|
ALZHEIMER’S DISEASE: ARE WE INTERVENING TOO LATE? NO WE ARE NOT!
D. Ames
National Ageing Research Institute and University of Melbourne Professor of Ageing and Health, Parkville, Victoria, Australia
Alzheimer’s disease (AD) represents a major public health challenge. It is the most common cause of dementia, whose worldwide prevalence doubles every 20 years for the foreseeable future. It would be good if it were possible to treat AD early in order to diminish its impact, but current available evidence does not support early intervention.
Vitamin E was no more effective than placebo in a study of Vitamin E and donepezil against placebo in mild cognitive impairment (MCI). Vitamin E is associated with a higher rate of hemorrhagic events than placebo. Neither donepezil nor galantamine has been shown to be helpful in retarding progression from MCI to AD.
Gingko biloba was not shown to be effective in delaying the onset of AD in a large prospective trial involving over 6000 participants.
Gamma secretase inhibitors have not yet been shown to retard the progression of AD and they seem to have a high incidence of adverse effects, especially rashes. Antibody therapy has not yet been shown to be helpful in the treatment of established AD let alone its prevention. Metaloproteinase modifiers such as PBT2 may be useful AD therapies, but current evidence gives no support to their immediate use in pre- symptomatic AD. No evidence can yet be adduced to support the use of antibody therapies in MCI or early AD.
For these reasons it is clear that the early treatment of AD cannot be justified as yet, no matter how desirable this goal may be. Treatment of established AD with cholinesterase inhibitors and memantine, coupled with referral of interested patients to evaluative drug trials is the best we can do at present.
DOPAMINERGIC MEDIATION FOR THE BRAIN PLASTICITY
O. Bajenaru
Romania
Traditionally in the clinical practice dopaminergic disturbances are related almost exclusively to movement disorders and sometimes to psychotic behavior. On this basis well-known drugs have been developed especially for Parkinson’s disease and psychotic conditions. During the last 15-20 years, the clinical observations of the patients treated with drugs influencing the dopaminergic systems of the brain have shown that in many instances together with the expected clinical improvement, sometimes important secondary unexpected clinical manifestations appear related to these drugs. These observations, together with the progress in understanding the pathophysiology of important clinical conditions such as Parkinson’s disease and other neurodegenerative diseases, led to the development of both clinical and experimental research on dopamine and dopamine receptors functions in different parts of the brain. More and more data concerning an important role of dopamine and its receptors in normal and pathological neuroplasticity of the brain are available today, so we consider that a synthesis on this topic could be interesting.
TREATMENT OF ANEURYSMAL SUBARACHNOID HEMORRHAGE: COILING
O. Bajenaru, F. Antochi, B. Dorobat, R. Nechifor, G. Iana
Romania
Although the most recent guidelines for the treatment of aneurysmal subarchnoid hemorrhage recommend both surgical clipping or coiling as therapeutic measures, without denying the value of clipping which is very laborious and has many limits, in our opinion and based upon clinical trials and our own experience during the last few years, we consider that in most clinical situations endovascular treatment is preferable. This opinion is supported by the fact that this type of cure is less invasive, does not require general anesthesia and is also suitable for patients in more critical situations when the “classical” surgical method for clipping is not recommended. The recent technological advancement allows even by combining stenting and coiling treating not only the aneurysms with a narrow neck but also those with a large neck, with good results. Also the endovascular approach allows in patients whom arterial vasospasm does not react to medical treatment with nimodipine, to solve it by local endovascular intervention. These are arguments to prove that in the hands of experienced specialists, the endovascular cure of cerebral aneurysms is a preferable method.
SHOULD MECHANICAL EMBOLECTOMY DEVICES BE USED IN ROUTINE CLINICAL PRACTICE? YES
M. Bar
Faculty Hospital, Ostrava, Czech Republic
The incidence of ischemic stroke in various European countries is between 183-349 /100 000 and in Czech Republic 219/100 000.
Acute occlusion of cervical or intracranial arteries is the most common cause of ischemic stroke (IS). A high rate of pathologic findings - occluded arteries was found in patients with acute stroke during the first 6 hours (77 % in NAIS study).
Clinical studies have shown that the prognosis of patients with occlusion of the intracranial arteries in the acute phase of ischemic stroke is worse when compared to patients without any occlusion. Timely recanalization of the occlusion is an important independent prognostic factor. Vice versa in MERCI trial the absence of recanalization was associated with higher mortality.
Overall the restoring of cerebral perfusion (that means in fact the vessel recanalization) within the first hours after stroke onset is currently only one effective treatment of acute ischemic stroke.
Currently, the following options for acceleration of recanalization of the intracranial artery occlusion are available:
• Intravenous tPA thrombolysis (IVT),
• Intra-arterial tPA thrombolysis (IAT)
• Mechanical recanalization using various devices (MERCI retriever L5, X6; Penumbra Stroke system; balloon angioplasty and stenting; EPAR; LaTIS laser device...)
• Intra-arterial tPA thrombolysis using EKOS System
• Combination of IVT and mechanical recanalization technique
• Sono-thrombolysis
Intravenous pharmacological recanalization is currently the most cost-effective and available treatment method in an acute IS nevertheless almost 50% patients die or remain disabled.
The restrictive inclusion criteria limit the utilization of intravenous tPA. Only 4.3 % patients with acute stroke in Czech Republic receive IVT. We achieve early recanalization in only 30-50% patients generally and even only 10% in distal carotid occlusion.
Mechanical embolectomy represents the chance to increase the number of patients with early recanalization.
The MERCI study tested whether mechanical embolectomy device is safe an efficient to open occluded intracranial vessel within 8 hours of the onset of stroke. Multi MERCI trial followed MERCI allows also the enrolment of patients after ineffective IVT. Recanalization rate was 53% with the device alone and even 73% after any adjunctive thrombolytic therapy. Successful recanalization resulted in functional independence in 47 % patients. In August 2004, the Merci Retriever was approved by the United States Foods and Drug Administration (FDA) to recanalize cerebral vessels in acute ischemic stroke patients.
Mechanical embolectomy is indicated:
• For patients up to 8 hours from stroke onset with severe neurological deficit (NIHSS >= 10)
• For patients with detected occlusion of major intracranial or cervical vessels
• For patients out of 4,5 hours window
• For patients within 4,5 hours time window but with IVT contraindications
• For patients who fail to recanalize after IVT.
Because recanalization rate after IVT is low in proximal large vessel occlusion some patients may have embolectomy even as the first option. The risk of mechanical embolectomy is related to intracranial hemorrhage or complications of endovascular devices (wall perforation or other mechanical damage). Recanalization in MERCI trial was achieved with an acceptable low rate of intracranial symptomatic hemorrhage (8-10 %; 2, 4% with PM-2). Device related complication occurred in 5.7%. Mechanical embolectomy is also more effective in removing large thrombi in proximal intracerebral arteries than IA thrombolysis.
Besides mechanical embolectomy by MERCI Retriever the angioplasty and stenting are also used successfully in acute ischemic stroke caused by hypoperfusion from stenotic intracranial or extracranial high-grade stenosis. At present day we have not any medical evidence of efficacy of angioplasty or stenting.
Despite of many limitations (cost, availability) mechanical embolectomy is the additional option in acute stroke care to increase the number of patients with early recanalization. That is truth that we have not data from randomized double blind studies. But we clearly know that prognosis of patients without recanalization of intracranial large vessel is very poor. The outcome is strongly dependent on time of recanalization. Besides the implementation acute stroke unit service and IV thrombolysis treatment in routine clinical practice the mechanical embolectomy represents other significant step to improve stroke care. European Stroke Organization guideline 2009 recommends intra-arterial treatment of acute MCA occlusion within a 6-hour time window as an option (Class II, Level B).
In my hospital we organize acute stroke care as following:
• All patients up to 24 hours from stroke onset are admitted to stroke unit.
• All patients up to 4.5 hours who fulfill indicated criteria are treated by IVT.
• Patients up to 4,5 hours who do not meet criteria for IVT and the others up to 8 hours with extra or intracranial large vessel occlusion are indicated to cerebral angiography.
• Patients who are treated by IVT are monitored during administration tPA by transcranial duplex sonography. In case on IVT failure patients are indicated to angiography too.
Personally I answer yes to question above and I agree with implementation of mechanical embolectomy to routine clinical practice. I do it in my hospital.
IS AED DISCONTINUATION IN SEIZURE-FREE PATIENTS DANGEROUS? NO
E. Beghi
Laboratory of Neurological Disorders, Istituto “Mario Negri”, Milano, Italy
Practicing physicians are still concerned about discontinuation of antiepileptic drugs (AEDs) in patients with prolonged seizure freedom. However, several reports provide fairly robust evidence in support of treatment discontinuation in these cases. A long-term population-based study has shown that 5-year terminal remission (i.e. off-drugs) of epilepsy is 61% (Annegers et al, 1979). These findings have been later confirmed by several other studies. In a systematic review of the literature, the relapse rate at two years was found to range from 43 to 65% in adults and from 9 to 39% in children (Specchio & Beghi, 2004). 2 randomized trials have assessed the effects of AED withdrawal on seizure relapse and patients’ performance. In the first trial (MRC Group, 1991), patients randomized to continued treatment showed a 22% relapse at two years, while patients randomized to slow drug withdrawal had 41% relapse. This differential risk of relapse was maximal between 1 and 2 years and declined thereafter. After 2 years, the risk of subsequent relapse was the same for both treatment groups. The risk of recurrence was also similar in patients who relapsed after withdrawal of AEDs and in those who relapsed while remaining on treatment (Chadwick et al, 1996). In the second trial (Lossius et al, 2008), 15% patients randomized to treatment withdrawal and 7% of those randomized to remain on treatment had a relapse at 12 months, a non-significant difference. However, compared to the latter, the former improved significantly in their neuropsychological performance. Several factors are associated with an increased risk of relapse after treatment discontinuation. These include the onset of seizures in adolescence, the presence of partial seizures, a documented etiology, and an abnormal EEG at the time of treatment discontinuation. Compared to patients with onset of seizures in childhood, those with onset in adolescence have a 1.8 chance of relapse. The risk of recurrence is 1.5 in patients with a documented etiology of seizures and 1.4 in those with abnormal EEG (Berg & Shinnar, 1994). This modest increase in risk is still compatible with treatment discontinuation even in patients with adolescent-onset of seizures, symptomatic seizures, and abnormal EEG. The duration of seizure freedom and the mode of withdrawal are other critical issues. However, in a Cochrane systematic review of studies done in children and adults, Sirven and colleagues (2001) found that the pooled relative risk for seizure relapse in early (less than two seizure free years) versus late (more than two seizure free years) AED withdrawal was 1.32 (95% confidence interval 1.02 to 1.70), s statistically significant but clinically irrelevant difference. In another Cochrane review, Ranganathan and Ramaratnam (2006) assessed the comparative effects of slow versus rapid AED withdrawal. Only one trial done in children satisfied the selection criteria: In this study, no differences were found in the risk of relapse comparing rapid (six weeks) to slow (nine months) taper group. However, in view of the methodological deficiencies and small sample size in the solitary study identified, the authors could not derive any reliable conclusions regarding the optimal rate of tapering of AEDs. In this light, ddiscontinuation of drug treatment is not dangerous and is a valuable option in patients with epilepsy who are seizure-free for two years or longer. The decision to withdraw or withhold treatment in these cases must be, however, individualized, subjected to the calculation of the risk of relapse after treatment stop and to the involvement of the patient in the decision process.
References: Annegers, JF, Hauser, WA, Elveback, LR. Remission of seizures and relapse in patients with epilepsy. Epilepsia 1979; 20:729 -737. Berg AT, Shinnar S. Relapse following discontinuation of antiepileptic drugs: A meta-analysis. Neurology 1994; 44:601-608. Chadwick D, Taylor J, Johnson, T. Outcomes after seizure recurrence in people with well-controlled epilepsy and the factors that influence it. Epilepsia 1996; 37:1043-1050. Lossius MI, Hessen E, Mowinkell P, et al. Consequences of antiepileptic drug withdrawal: A double-blind, randomized study (Akershus Study). Epilepsia 2008; 49:455-463. MRC Antiepileptic Drug Withdrawal Group. Randomized study of antiepileptic drug withdrawal in patients in remission. Lancet 1991; 337:1175-1180. Ranganathan LN, Ramaratnam S.Rapid versus slow withdrawal of antiepileptic drugs. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No.: CD005003. Sirven J, Sperling MR, Wingerchuck DM. Early versus late antiepileptic drug withdrawal for people with epilepsy in remission. Cochrane Database of Systematic Reviews 2001, Issue 3, Art No CD001902. Specchio LM, Beghi E. Should antiepileptic drugs be withdrawn in seizure-free patients? CNS Drugs 2004; 18:201- 212.
SHOULD PROPHYLACTIC ANTIEPILEPTIC THERAPY BE PRESCRIBED TO PATIENTS WITH BRAIN TUMORS? NO
E. Beghi
Laboratory of Neurological Disorders, Istituto “Mario Negri”, Milano, Italy
Epilepsy is a common complication of brain tumors and seizure control is an important part of clinical management. Given the frequency of epilepsy in patients with brain tumors, prophylactic use of antiepileptic drugs (AEDs) is common in clinical practice. However, the results of studies on the effects of prophylactic use of AEDs are conflicting. Three meta-analyses of studies done in patients with brain tumors and no seizures (Glantz et al, 2000; Sirven et al, 2004; Tremont-Lukats et al, 2008) failed to suggest efficacy of AEDs in preventing a first seizure. In addition, Tremont- Lukats and coworkers found a sixfold risk of adverse events in treated individuals compared to those who were left untreated (number-needed- to-treat, NNT 3). In addition, a number of adverse treatment events have been observed in patients receiving AEDs (van Breemen et al, 2007). These include cognitive impairment, bone marrow suppression, liver dysfunction, and dermatologic reactions. These adverse events tend to prevail in patients with brain tumors because of ageing, gastric mucosal atrophy, higher fat-to-lean body mass ratio, declining liver and/or kidney function, which may lead to an impaired pharmacokinetics of the available compounds. Finally, interactions between AEDs and antineoplastic agents may lead to inadequate control of the underlying tumor or epilepsy as a consequence of enzyme induction, leading to faster metabolism and lower plasma concentrations of agents sharing the same isoenzyme. This mechanism explains the decrease in the effectiveness of corticosteroids and several chemotherapeutic agents. AEDs inducing cytochrome P450 coenzymes include phenobarbital, primidone, carbamazepine and phenytoin (drugs commonly used by neurosurgeons). In contrast, valproate is an enzyme-inhibiting drug which may raise the plasma concentrations (and the toxicity) of other compounds. Altered phenytoin concentrations have been also observed in patients receiving dexamethasone.
For these reasons, the need for anticonvulsant treatment for seizure prophylaxis before or after surgery is uncertain and there are few data in support of practical guidelines for prophylactic use of AEDs in patients with brain tumors.
References: Glantz MJ, ColeBF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neurology 2000; 54:1886-1893. Sirven JI, Wingerchuck DM, DrazkowskiJF, et al. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin Proc 2004;79:1489-1494. Tremont -Lukats IW, Ratilal BO, Armstrong T, Gilbert MR. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No.: CD004424. Van Breemen MSM, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007;6:421 -430.
SUBSTANTIA NIGRA HYPERECOGENICITY IS A RISK MARKER FOR PD: YES
D. Berg
Center of Neurology, Department of Neurodegeneration and Hertie-Institute of Clinical Brain Research, University of Tübingen
Clinical as well as histopathological studies give evidence for an ongoing neurodegenerative process, most likely many years before the typical motor symptoms allow the clinical diagnosis of Parkinson’s disease (PD). To allow an earlier, disease modulating or even neuroprotective therapy it is important to identify subjects in this state, which means at a time before the majority of dopaminergic neurons of the substantia nigra are lost. During this phase specific non-motor symptoms like hyposmia, depression, RBD, slight neuropsychological deficits, autonomic dysfunction and others can occur.
In recent years, transcranial sonography (TCS) has evolved as a useful instrument in the differential diagnosis and even very early diagnosis of PD. As a typical hallmark, hyperechogenicity (an enlarged and more intense signal) at the anatomical site of the substantia nigra (SN) can be found.
Importantly this ultrasound signal can also be detected in about 10% of healthy subjects. There is accumulating evidence that SN hyperechogenicity may disclose a vulnerability of the nigrostrial system in at least some of these persons. Moreover, an association of the ultrasound sign with a number of risk and premotor markers has been shown.
According to these studies subjects with SN hyperechogenicity but without the clinical picture of PD may show
(i) a reduced presynaptic tracer uptake in PET and SPECT examinations of the nigrostriatal system in about 60%,
(ii) signs of motor retardation, sole resting tremor and other slight extrapyramidal symptoms with increasing age,
(iii) more often and more severe extrapyramidal features following administration of neuroleptics,
(iv) unilateral motor slowing when performing demanding motor tasks,
(v) more often premotor symptoms, like
a. olfactory dysfunction,
b. depression,
c. specific neuropsychological deficits like visuospatial processing and sequential planning, known to be primarily affected in PD,
d. RBD than healthy subjects without this ultrasound signal.
Moreover, asymptomatic mutation carriers for monogenetically caused PD reveal SN hyperechogenicity, in some instances even prior to detectable deficits in PET-examinations.
However, the most striking argument of a relevance of SN hyperechogenicity in healthy subjects can be derived from the observation that since the beginning of the examination technique at least 14 initially healthy subjects with SN hyperechogenicity have been observed to develop PD in follow up investigations by the authors (unpublished data).
Based on these findings, large longitudinal studies are being performed to evaluate the prospective value of the ultrasound sign in delineating a subgroup at high risk for PD. Already the baseline investigations of these studies show that healthy subjects older than 50 years show more often a combination of several premotor and risk markers for PD than persons without this echosign.
There are limitations to the method like an insufficient bone window in about 10% of elderly subjects. Also, the method requires some experience to delineate brain structures, similar to the expertise required for the interpretation of the results of other neuroimaging techniques. Still, if also the longitudinal studies confirm that TCS contributes to the premotor diagnosis of PD, this method could serve as an excellent screening tool due to its broad availability, short duration of investigation, possible application even in agitated subjects and low costs.
TEMPERATURE AND BAROMETRIC PRESSURE ARE THE MOST SIGNIFICANT CONDITIONS FOR TRIGGERING MIGRAINE HEADACHE: CON
H. Bolay
Department of Neurology & Neuropsychiatry Centre, Gazi University, Ankara, Turkey
Migraine headache is triggered by various extrinsic and intrinsic factors. One of the environmental factors is weather conditions, and studies have revealed that 7–43% of patients are susceptible to atmospheric changes. Several studies have shown that atmospheric conditions can affect well-being and, particularly, headache/migraine is reported to be most frequent symptom (61%). Atmospheric weather changes including, pressure, humidity, wind and temperature were all implicated as a potential trigger for headache, though a definite link could not be demonstrated. Low barometric pressure and low temperature have been associated with increased pain intensity in humans as well as experimental animals. Emergency department visits for headache were correlated with air pollution-related increases in levels of SO2 (sulphur dioxide), NO2 (nitrogen dioxide), CO (carbon monoxide) and PM 2.5 (particulate matter i2.5 im).
We recently defined a novel atmospheric factor as a potential trigger for migraine by demonstrating the effect of Saharan desert dust- laden atmospheric conditions on trigeminovascular system activation. One-–1.5 billion tons of dust are thrown from the Saharan desert and scattered to the atmosphere each year. Fifty to 75 % of the dust transported all around the globe originates from Africa. Besides closer continents such as Europe and Asia, Saharan desert dust effects transatlantic regions, primarily the Caribbean and America, including the USA and beyond. It has been recently noticed that dust originating from deserts has an adverse effect on public health, such as increased asthma attacks. Dust originating from the Saharan Desert and attached microorganisms such as bacteria, viruses, fungi and their spores could multiply and initiate a series of reactions upon contact with cloud water and solar energy resulting in the formation of reduced iron (Fe++), oxalate and various basic amino acids.
The simulation of Saharan dust-containing atmospheric conditions was examined in freely moving rats. Inhalation of air containing African dust induced significant c-fos expression in brainstem nociceptive neurons within trigeminal nucleus caudalis. However, when the same African dust was used in the experiments after treated with Co60 gamma ray to kill all living microorganisms, c-fos expression was not detected. That result showed that nociceptive effect was carried by microorganisms attached to the dust. To further evaluate the direct or indirect role of microorganisms, we used several filters for particles and concluded that the effect was mediated by particles less then 500 im, far smaller than any living virus or bacteria. Atmospheric samples were also analyzed for microorganisms.
We suggest that the nociceptive effect was unlikely mediated directly by microorganisms rather their by products such as basic amino acids, Fe++, oxalate generated during the transportation of Saharan dust in the atmosphere where sun light and water from cloud was easily accessible and the microorganisms began multiplying. This previously unknown novel factor in the atmosphere is exciting hypothesis as trigger for headache. Clinical prospective studies on the potential triggering effect of Saharan dust-containing weather alterations on patients with migraine and other headaches could provide notification of patients for preventive medication on a daily or seasonal basis. Beside other parameters the African Dust content is a very important atmospheric factor for triggering headache.
THE ORIGIN OF MIGRAINE ATTACKS: CORTICAL SPREADING DEPRESSION IS THE FIRST PHASE OF EACH MIGRAINE ATTACK
H. Bolay
Department of Neurology & Neuropsychiatry Centre, Gazi University, Ankara, Turkey
Migraine attack is generated by the complex interaction of various players such as genetic predisposition, environmental and intrinsic factors. However in quest of the origin of a migraine attack is challenging task and the scientific evidences so far indicate cortical spreading depression (CSD), as the most likely initiating event. In my talk I will provide research data that demonstrates the involvement of SD in migraine headache.
CSD indicates an extreme excitability state of the gray matter with massive redistribution of ions that emerges in reaction to noxious stimulus. CSD is the pathophysiological event underlying migraine aura and occurs in different clinical conditions such as cerebral ischemia, head trauma or subarachnoid hemorrhage. CSD triggers various neuronal and vascular changes in brain parenchyma as well as in the meningeal membranes called pia, arachnoid and dura mater. During CSD, a brief hyperperfusion followed by a prolonged oligemia is associated with DC shift and propagates over cerebral cortex (without necessarily matching any vascular territory). CSD, an intense intrinsic brain event is also able to induce trigeminovascular activation that is a characteristic feature of headache phase. CSD induce neurogenic blood flow increase, vasodilatation and neurogenic plasma protein extravasation in the overlying dura mater. CSD also triggers c-fos induction in the ipsilateral trigeminal brain stem nuclei (TNC).
Recent genetic and pharmacological findings are also supportive of important role of CSD in migraine. Otosomal dominantly inherited form hemiplegic migraine is caused by mutation of ion channels or transporters such as CACNA1A and SCNA1 or Na+-K+ ATPase, in a way that results in release of excessive glutamate from neurons, reduced uptake of glutamate from the synaptic cleft into glia, and/or reduced buffering capacity to potassium ions. The common result of all three identified mutations is the hyperexcitability and reduced threshold for CSD induction, which all probably contribute to the vulnerability of the brain to migraine attacks. From the therapeutic perspective, the efficacy of certain anti-epileptic drugs in migraine patients and their action on excitability or even on CSD is noteworthy.
In that sense the demonstration of hyperexcitability and sustained increase in the efficacy of synaptic transmission in the affected neocortex as a long-term complication of CSD in human brain tissues is remarkable. In addition the brainstem that is resistant to SD waves, were shown to be easily transformed in to a structure vulnerable to SD with the accurate priming stimuli. It was notable that such a switch to vulnerable state was reversible and how extrinsic or intrinsic triggering factors modulate the vulnerability and interact with ionic gradient forces across the cell membrane to surmount histomorphological resistance to SD remain unknown. Impaired neurovascular coupling associated with CSD was reported. CSD in lissencephalic brain was demonstrated to induce significant redox state in the cerebral tissue that was improved by increased O2 supply. CSD per se was shown to induce insufficient glucose supply for as long as 30 minutes in gyrencephalic brain. Emerging of CSD probably induce a vicious circle where long-term enhanced synaptic efficacy and increased excitability renders the cortical tissue for the next CSD , particularly in the setting of metabolic compromise such as hypoxia and decreased glucose supply.
In conclusion, SD in cerebral cortex or other subcortical/ brainstem structures with varying speed and distribution leading to overt or silent symptoms could be the only accountable explanation for a migraine attack.
THE FERTILE SOIL OF ALIENATION
R. Bondy
Ramat Gan, Tel Aviv, Israel
The title was chosen on the spur of the moment, it should have been "The fertile soil of tension between alienation and belonging", because living in Bohemia and Moravia, the Czech crown lands, more than one thousand years, the Jews felt belonging, to the countryside, the hills, the rivers, the villages, the towns, to Prague, called by them עיר ואם, city and mother, even when mother had claws which did not let go, as Kafka wrote.
It was a belonging on condition, till the next expulsion, the next riot, with all the restrictions of residence, the prohibition to work the soil, to make a living as artisans outside of the ghetto, and may be the harshest of all: to multiply. From 1726 the number of Jewish families was restricted to 8541 in Bohemia and 5106 in Moravia. Only the first born son could inherit the family number, marry and have children. The other sons had to stay single, immigrate to countries where the Familianten law did not exist or marry clandestinely by Jewish rite, but then the children were registered as illegitimate and the couple and the rabbi who married them were always in danger of severe punishment.
The Ukrainians killed Jewish children in progroms, the Hapsburg monarchy did it by sheer bureaucracy, now known as Kafkaesk, Kafka, Freud, Mahler, to name only the outstanding geniuses, were born in the Czech crown lands after the Jews gained 1849 the right to multiply without restriction, otherwise they may have never existed.
Even after abolishing the Familianten law, the number of Jews in Bohemia and Moravia remained small, 120,000 in 1930, around 1.1% of the population. The question is: how come, that from such a negligible number of Jews, not more than the population of a small town, grew some of the greatest minds of the 20th century, as well as many outstanding writers, scientists, philosophers, architects, artists, musicians? Was it by mere chance or was there something in their tradition, background, upbringing, or surroundings that predisposed them to excel? For sure, I am not objective and prefer the second possibility.
In my lecture I shall try to find some of the answers, leaning on their biographies.
*Growing up bi-lingual, German being the language of their education, their cultural orientation, Czech the language all around them, learning from early childhood that the same object can have different names – Tisch or stul, Gabel or vidlicka – opens the mind to nuances, possibilities.
*The humble background of their fathers, many of them peddlers with a bundle on their back, who lived frugally and saved penny to penny to enable their sons to study and have a better future.
*Alienation of the studied sons from their uncouth fathers and their reminiscences, with no father figure to lean upon. They had to find their own way.
*Their migration in the second half of the 19th century from villages to towns and especially to Prague and from there to Vienna to succeed, and, if necessary for a higher position, their willingness to get baptized (Mahler).
*Being on the move made them receptive to progressive ideas, liberalism in the 19th century, communism in the 20th century, out of the belief that it really meant equality and brotherhood.
*The Czechs were more free-thinking and less religious than other Slavic nations, influenced by the teaching of Jan Hus, the reformer, and the Jews in their midst were influenced by them and cut themselves loose from religious tradition earlier than the Jews in the surrounding countries. They had to find their raison d'etre of their own.
*With all their secularization, the thousand years of Jewish learning, of erudition were deeply engrained in them, and as soon as the Charles University in Prague, closed to them for centuries, admitted Jewish studies, they seized the opportunity: in 1900 almost 30% of the students there were Jewish.
*Even if their German and Czech was perfect, even if they longed for it, they could never become real Germen, Austrians, or Czechs in the eyes of their compatriots, who saw them first and foremost as Jews. They remained outsiders, they got used to seeing things as onlookers, to analyze.
*Even the successful and well situated Jews could never feel secure, were prone to anxieties. They never could sit back and relax – they did not know how.
CT BEFORE LP IN SUSPECTED BACTERIAL MENINGITIS
I. Bone
Dept of Medical and Cardiovascular Studies Western Infirmary Glasgow, Scotland, UK
Background: Few would argue that cranial neurological disease should be investigated by imaging and that this, in critical neurological illness, be an early investigation. Historically lumbar puncture has played a major, though now diminishing role in most investigative protocols. The risk of lumbar puncture in the context of raised intracranial pressure (ICP) is known and accepted. Brain herniation syndromes can develop, are often delayed, mistaken for disease progression and in survivors can lead to catastrophic outcomes such as cortical blindness from posterior cerebral artery territory infarction. Why chance this? No matter how small the risk, identification of a mass lesion by imaging may prevent a life threatening LP. Raised intracranial pressure occurs in meningitis without a discrete mass lesion being present. A “tight brain” with obliteration of sulci and cisterns can be detected by CT. Is this not also at risk from LP? Finally where there is diagnostic certainty how necessary in any case is an LP? For this debate we will assume the availability of 24hrs CT, an ability to interpret imaging and a high degree of diagnostic certainty of acute bacterial meningitis (ABM). Where CT is not immediately available or the skills to interpret absent, risks and benefits weigh differently and pragmatism prevails.
The case for LP before CT in selected cases: Because of the potential risks of lumbar puncture, CT of the head is widely used to identify patients in whom lumbar puncture should be avoided. A study of 301 adults with suspected meningitis to determine whether clinical characteristics, present before CT of the head was performed, could be used to identify patients who were unlikely to have abnormalities on CT. 235 (78 percent) underwent CT of the head before undergoing diagnostic lumbar puncture. In 56 of the 235 patients (24 percent), the results were abnormal. 11 patients (5 percent) had evidence of a mass effect on CT of the head. Importantly the authors noted a trend toward a longer time from admission to the initiation of empirical antibiotic therapy in those who underwent CT before lumbar puncture. They claimed that clinical features can predict those with mass effect and that the decision for CT before LP should be electively made on such grounds. Others have reached similar conclusions. Delay in initiating antibiotics is an accepted predictor of poor outcome and a policy of “imaging all” suspected cases could have such a consequence. Finally a literature review found no cases of patients with acute meningitis deteriorating as a result of lumbar puncture.
The Case for CT before LP in all cases: The studies supporting selective imaging on clinical grounds alone have been carried out at single institutions following a research protocol using assessments such as the NIHSS. These studies show a significant number of CT abnormalities with 1 in 20 persons having mass effect. The stringent clinical assessment offered to patients in these studies could not be guaranteed in the average ER room in an “out of hours” situation. Several assumptions are made by those who argue against immediate imaging. Firstly that the process takes up valuable time. This is not borne out by the experience emergent imaging for acute stroke prior to thrombolysis. Secondly that immediate antibiotic therapy, on first clinical suspicion, will affect the validity of subsequent lumbar puncture bacteriology.
An LP before imaging can have an effect upon subsequent imaging. Whilst meningeal enhancement following LP is infrequent it can lead to subsequent diagnostic difficulties. Many imaging studies were targeted at excluding mass lesions rather than features of raised ICP. An early CT study addressed the anatomical criteria that correlate with unequal pressures between intracranial compartments and predispose a patient to herniation following decompression of the spinal compartment (LP): these were lateral shift of midline structures, loss of the suprachiasmatic and basilar cisterns, obliteration of the fourth ventricle, or obliteration of the superior cerebellar and quadrigeminal plate cisterns with sparing of the ambient cisterns. These simple criteria may predict those in whom an LP might be unsafe. Joffe claims that brain herniation occurs in about 5% of patients with ABM, accounting for about 30% of total mortality. Whilst he advocates early CT he cautions that “a normal scan in does not mean that an LP is safe”. Joffe suggests that the clinical signs of "impending" herniation are best predictors of when to delay an LP. These being deteriorating level of consciousness (GCS <11), and evolving brainstem signs. In those at high risk for brain herniation, he recommends that interventions to control intracranial pressure should be the priority, followed then by an urgent CT scan and not an LP.
Conclusion: Why should ABM be an exception to the rule that emergent brain imaging is mandatory in all acute central nervous system infections? LP before CT implies brain imaging is unnecessary in such cases unless there is further clinical progression. Whilst some claim that brain herniation following LP is rare, and can be predicted on clinical grounds alone, the evidence supporting this stance is unsafe. Brain herniations occur some time (hours) after LP and can be mistakenly attributed to the underlying illness itself rather than the diagnostic intervention. Anecdote should not form the basis of clinical practice but many neurologists have had the experience of deterioration “on the end of a needle” and see this as a preventable disaster. Where imaging is available and evidence questionable caution must prevail. Mechanistically brain herniation following LP is those with raised ICP is plausible. Radiological signs for those at risk, in conjunction with clinical assessment, should be applied to all with ABM before LP is considered.
References: 1. Hasbun R, Abrahamj, Jekel.J, et al. Computed Tomography of the Head before Lumbar Puncture in Adults with Suspected Meningitis 2. Baker ND, Kharazi H, Laurent L, et al. The efficacy of routine head computed tomography (CT scan) prior to lumbar puncture in the emergency department. J Emerg Med 1994; 12:597-601 3. Proulx, N., Frechette, D., Toye, B., Chan, J., Kravcik, S. (2005). Delays in the administration of antibiotics are associated with mortality from adult acute bacterial meningitis. QJM 98: 291-298NEJM. 345:1727-33 December 2001 4. Archer.B.D. Computed tomography before lumbar puncture in acute meningitis: a review of the risks and benefits. Canadian Medical Association Journal, 1993 Vol 148, Issue 6 961-965 5, Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale: extension to non-neurologists in the context of a clinical trial. Stroke 1997; 28:307-310 6. Mittl RL and Yousem DM Frequency of unexplained meningeal enhancement in the brain after lumbar puncture. American Journal of Neuroradiology, 1994; 1:4 633-638 7. Gower D J, Baker AL, Bell WO and Ball MR Contraindications to lumbar puncture as defined by computed cranial tomography. Journal of Neurology, Neurosurgery, and Psychiatry 1987; 50:1071-1074 8. Joffe.AR Lumbar Puncture and Brain Herniation in Acute Bacterial Meningitis: A Review Journal of Intensive Care Medicine, Vol. 22, No. 4, 194-207 (2007).
IS SPECT IMAGING CRITICAL TO DIAGNOSE DEMENTIA WITH LEWY BODIES? NO
M. Bojar
Charles University in Prague, 2nd Medical School, Department of Neurology, University Hospital Motol, Prague, Czech Republic
Dementia with Lewy bodies (LBD) has been attracting attention of various medical specialities since 1996 when the diagnostic criteria for LBD were established by Mc Keith et al. Due to arying data on prevalence of the enigmatic LBD, various clinical manifestation, relationship to Alzheimer´s Disease as well as prof. Korczyn´s hypothesis of the possible continuum of Alzheimer´s –Parkinson´s Disease and LBD, alarming reports on lethal complications caused by neuroleptics used to sedate restless LBD patiens, the search for an effective diagnostic method has been very intensive. Since structural imaging methods are not effective in LBD diagnosis very high expectations have been associated with functional neuroimaging methods. However, the effectivity of a commonly recommended and frequently used new diagnostic tool- DaTSCAN- is limited and its clinical yield should not be overerestimated. The standard and traditonal clinical approach based on anamnestic data, precise, old-fashioned neurological and psychiatric examination and thoroughful follow-up of the suspected LBD patients play the key role in detecting possible LBD patiens. This komplex approach can not be replaced by a High-Tech neuroimaging method. Clinical examination of suspected LBD subjects and analysis of data should always preceed the DaT SCAN, which is not critical for the diagnosis of LBD and can not be used alone effectively for screening or for prima vista diagnosis of LBD. The sensitivity of DaT SCAN to detect clinical probable DLB was 78% and the specificity to exclude non LBD dementia exceeded 90% in a Mc Keithś multicentric study analysing data on probace/possible LBD in LBD,Parkinson´s disease and dementia 326 patients. The sensitivity/specificity ratio as well as the price of DaTSCAN which used to be classified as satisfying or very high however, 13 months after the big NY LB Collapse when facing the thread of another big collapse – the HIC and PHS collapse in some EU countries - are very strong arguments not to classify DaT SCAN as critical, but only useful to diagnose LBD.
LAQUINIMOD: AN ORAL IMMUNOMODULATING BREAKTHROUGH IN THE TREATMENT OF RELAPSING-REMITTING MULTIPLE SCLEROSIS
A.N. Boyko
Department of Neurology and Neurosurgery, Russian State Medical University, Moscow MS Center, Russia
Laquinimod is a novel oral, once-daily immunomodulator which is currently being developed as a treatment for relapsing-remitting multiple sclerosis (RRMS). Laquinimod was demonstrated to be effective in various experimental autoimmune encephalomyelitis models. The ameliorating activity of laquinimod is mediated by various effects, including reduction of leukocyte infiltration to the target tissues, modulation of cytokine balance, down regulation of antigen presentation as well as pro-inflammatory gene expression (MHC class II), and neuroprotective effects as demonstrated by reduced spinal cord demyelization and axonal loss. The effects of oral daily 0.3 and 0.6 mg laquinimod on MRI-monitored disease activity were assessed in a 36- week, double-blind Phase II placebo-controlled study. The 0.6mg dose showed a robust effect on MRI parameters [50% reduction of mean cumulative number (weeks 12-36) of new-T2 lesions (p=0.0001) (55% effect on median number) as well as a 50% reduction in the mean cumulative number (weeks 24-36) of new T1-hypointense lesions (p=0.0064)]. The 0.6mg dose also showed a 33% reduction of annualized relapse rate (p=0.0978). The 0.3mg dose did not show an effect on MRI lesions in this study. The rate of adverse events was similar in all treatment groups. Liver enzymes were elevated in a dose-dependent manner, reversible in all cases, without accompanying bilirubinemia. The efficacy profile was reproduced and sustained in an additional 36-weeks double-blind active extension study and there were no new safety signals.
Based on these encouraging results, two global phase III studies with 0.6mg laquinimod in RRMS patients were launched and by now have completed enrollment of more than 2200 patients worldwide.
ANTI-ANGIOGENIC THERAPY FOR MALIGNANT GLIOMA: UNSUBSTANTIATED ENTHUSIASM VS. A MIRACLE TREATMENT
D. Blumenthal
Director of Neuro- oncology Service, Tel-Aviv Sourasky Medical Center
Median overall survival for glioblastoma (GBM) ranges from 10-18 months. Median survival is doubled by standard radiation therapy (Walker), and the addition of chemotherapy plays a role in further enhancing longevity. A meta-analysis of 3000 patients on 12 randomized trials of chemotherapy showed an increase of survival of 2 months in those treated with chemotherapy (Stewart). There is significant variability in individual responses to treatment. Recently, certain tumor molecular characteristics have been recognized as important prognostic and predictive factors for treatment response, specifically, the MGMT enzyme which enhances tumor response to radiation and chemotherapy when its promoter region is methylated (Hegi). The Phase III EORTC trial of RT + Temozolomide (now adopted as standard first-line therapy) showed a 2.5 month significant increase in survival (14.5 mo) from 12 mo with RT alone; 26% 2-year survival with concurrent chemotherapy (10% RT alone) (Stupp, NEJM 2005).
Recurrent GBM (rGBM) fares worse than newly diagnosed, with median survival of 3-6 months, 6-month PFS 15-25%, and response rates of approximately 10-15%. (Yung; Ballman)
Antiangiogenic therapy is based on the premise that GBM is a highly vascular tumor, with over-expression of angiogenic factors, namely vascular endothelial growth factor (VEGF). Bevacizumab is a humanized monoclonal antibody that inhibits VEGF. It has been FDA- approved for used with chemotherapy agents as first or second line therapy in metastatic colon cancer, and in locally advanced or metastatic non-small cell lung cancer. It has most recently been approved for the indication of recurrent/progressive GBM (FDA May 2009).
The growing data with Bevacizumab for rGBM shows response rates of 50-60%, with PFS-6 of 46%, and median survival of 42 weeks (Vredenburgh), clearly an improvement from the experience with standard chemotherapy agents for relapsed/progressive GBM.
Friedman and Cloughsey published results last month (JCO Sept 09) of a randomized phase II Bev vs Bev + CPT-11: PFS-6: 42.6, 50.3; response rates 28.2 and 37.8%; med OS 9.2 mo and 8.7 mo.3/167 patients had intracerebral hemorrhage (1.7%), grades 1, 2, and 4.
The majority of patients with rGBM who are treated with Bevacizumab show an initial radiographic improvement: volume of enhancing tumor decreases, mass effect and edema decrease, and often the patient shows concomitant clinical improvement. Whether these changes are due to antineoplastic response or just edema control is not completely clear. There appear to be a range of responses to treatment, from remarkable early improvement (after 1-2 treatments); to tumor progression and clinical decline within a few months after initial response; to a minority with a durable, long-term response.
The data to date support the use of anti-angiogenic therapy as an important treatment option which prolongs life, improves quality of life, and is overall well-tolerated for individuals with rGBM.
BRIVARACETAM
M. Brazdil
Brno Epilepsy Center, Department of Neurology, St. Anne’s University Hospital, and Faculty of Medicine, Masaryk University, Brno, Czech Republic
Brivaracetam (UCB 34714), the 4-n-propyl analogue of levetiracetam, is among the first clinically effective anticonvulsants to be discovered by optimization of pharmacodynamic activity at a molecular target. It possesses a binding affinity for the targeted synaptic vesicle glycoprotein 2A (SV2A) ten-fold above that of levetiracetam and also exhibits an ability to inhibit voltage-gated sodium channels.
In various experimental models of both acquired and genetic epilepsy brivaracetam revealed higher potency and efficacy than levetiracetam as an anti-seizure and anti-epileptogenic agent, and a wide therapeutic index. Animal and preclinical human studies demonstrated a favorable tolerability profile of brivaracetam. Treatment adverse events were mild to moderate, mostly of CNS origin, and resolved within 24 hours, with decreasing incidence after repeated intake.
In April 2009 UCB announced results from three Phase III clinical studies (N01252/1253/1254) assessing the efficacy and safety of brivaracetam as adjunctive treatment of partial seizures in adult epilepsy patients. Study N01253 achieved statistical significance in its primary efficacy endpoint, showing that adjunctive treatment with brivaracetam was associated with significant reductions in seizure frequency versus placebo. Study N01252 did not achieve statistical significance on the primary efficacy endpoint. Data from all three studies (N01252/1253/1254) confirmed that brivaracetam was generally well tolerated, with the majority of adverse events reported being mild to moderate in nature. On the other hand further detailed analysis of both the primary and secondary endpoints of these studies is needed.
Taking together all the available data, brivaracetam seems to be an extremely promising novel anticonvulsant which might bring in the future renewed hope for the patient with refractory epilepsy.
MECHANISMS OF DRUG RESISTANCE
M. Brodie
Epilepsy Unit, Western Infirmary, Glasgow, Scotland, UK
Around 30% of adults remain refractory to antiepileptic drug (AED) therapy despite having similar seizure semiologies and epilepsy syndromes to drug responsive individuals. Thus some patients with relatively benign syndromes like juvenile myoclonic epilepsy will become pharmacoresistant, whereas others with potentially more severe underlying substrates, such as mesial temporal lobe epilepsy associated with hippocampal sclerosis, will not have a further seizure after taking the first dose of their first ever AED. How, when and why do people with epilepsy become pharmacoresistant?
Observations from the Glasgow database of newly diagnosed adult patients receiving their first ever drug at the Epilepsy Unit and reviewed for up to 25 years in the same clinic have provided useful insights into the natural history of treated epilepsy. Prospective analyses of this expanding cohort were undertaken in 1997 (n=470), 2003 (n=780) and, most recently, in 2008 (n=1098). Overall, around 50% of patients became seizure free on their first ever AED with diminishing numbers responding to subsequent regimens either as monotherapy or in low dose combinations. Around 25% of the complete Glasgow population never had useful period of seizure freedom despite receiving many AEDS singly and in combination. Interestingly, a similar number never had another seizure after taking the first dose of their first ever AED.
Differences between these clinical phenotypes has included higher seizure densities prior to initiation of therapy and concurrent or previous psychiatric comorbidities suggesting greater underlying brain dysfunction in these individuals. The presence of a family history of epilepsy and the development of febrile convulsions also correlate with pharmacoresistance implicating a genetic component to suboptimal drug response. The recent analysis of 1098 adolescent and adult patients with newly diagnosed epilepsy showed that 68.4% were currently in remission with 31.6% remaining refractory to an increasingly wide range of AEDs.
Some putative factors underlying the development of “refractoriness” will be discussed. These include insufficient mechanistic diversity of AEDs, localized overexpression of efflux drug transporter proteins in the brain, changes in the properties of ion channels and receptors in human surgical brain tissue, and genetically determined factors affecting drug response.
The overall outcome in adult epilepsy has modestly improved over recent years as newer drugs with novel mechanisms of action have become generally available. There seems little doubt, however, that refractory epilepsy is associated with a localized overexpression of drug transporter proteins in astrocytes and capillary endothelium at the region of the epileptic focus, which actively extrude AEDs from their intended site of action. Similarly there are changes in the properties of pharmacological targets, particularly Na+ channels and GABA receptors, in brain tissue obtained surgically from patients with refractory epilepsy. Whether they are the cause or the result of the problem is not clear.
It is likely that refractory epilepsy is associated with complex genetic differences between responders and non-responders to AEDs. However, the observation implicating C3435T polymorphisms in the ABCB1 gene encoding p-glycoprotein is implicated in refractoriness has been called into question as increasing numbers of studies have not replicated the original observation. It is likely that single gene polymorphisms represent too simplistic a paradigm and we now need to await whole genome typing to confirm or refute this hypothesis. In addition it is essential that identical phenotypes are included in comparative studies.
New paradigms are needed for treatment strategies in patients with refractory epilepsy based on a better understanding of the various neurobiologies underpinning pharmacoresistance. We need to turn our face away from empiricism and develop a more scientific approach to management, including targeted pharmacotherapy for individual patients. To do this we need to understand better the neurobiologies underpinining the development and progression of seizures.
PYM50028 (COGANETM) IS A SMALL MOLECULE INDUCER OF NEUROTROPHIC FACTORS WITH POTENTIAL TO PROVIDE DISEASE MODIFYING BENEFIT IN PARKINSON’S DISEASE
J. Brotchie
Toronto Western Hospital, Toronto, ON, Canada
Much preclinical work has validated the concept of employing neurotrophic factors, especially GDNF and BDNF, as a potential means of delivering neuroprotective and neurorestorative benefit in Parkinson’s disease (PD). However, historically, attempts to deliver neurotrophic factors by infusion of protein or gene delivery to the brain have failed to show efficacy in controlled clinical studies. Cogane™ is an orally active drug that enters the brain and induces the production of BDNF and GDNF, thus having the potential the bypass the challenges associated with brain delivery of neurotrophic factors themselves. Cogane™ is a single chemical entity (PYM50028) with molecular weight 416.64.
In vitro, Cogane™ provides protective and restorative benefit to dopaminergic, as well as other classes of, neurons. In several studies in the MPTP lesioned mouse model of PD, Cogane™ consistently shows an ability to protect against, and restore, losses of dopaminergic function induced by MPTP. Importantly these actions are seen as both actions to maintain cell body function in the substantia nigra as well as dopaminergic terminal activity in the striatum. An investigative study, demonstrated an ability of Cogane™ to restore motor function in the MPTP-lesioned macaque model of PD, even when treatment was initiated over one year after the last MPTP administration. Further in-depth studies are in progress and will be reported by the end of 2009.
Cogane™ has undergone the standard safety and toxicological package required of pharmaceuticals and is ready for long-term clinical studies. Several Phase I clinical trials show Cogane™ to be safe and well -tolerated in man for up to 3 months. An ongoing Phase I study in healthy volunteers and PD patients is designed to define dosing regimens associated with plasma levels that provide efficacy in preclinical models. It is anticipated that this study is a prelude to proof of concept clinical studies to commence in 2010.
CAN WE PREVENT MS WITH EARLY LIFE VITAMIN D SUPPLEMENTATION AND EBV VACCINIATION: NO
J. Chapman
Department of Neurology, Sheba Medical Center, Tel Aviv University, Israel
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system and its etiology and pathogenesis are largely unknown. The geographic distribution of MS has suggested the influence of environmental factors prominent amongst which have been physical factors such as sunlight and viruses. Sunlight influences levels of vitamin D, low levels of which have been reported in MS and viruses such as EBV have been implicated in the disease. There are a number of issues that argue against these factors being significantly modified as a form of MS prophylaxis:
EBV persistent infection is associated with lymphocytes including those infiltrating the CNS and therefore much of the data implicating the virus may be a by product of inflammation. Early infection with viruses such as EBV may actually be protective. Vaccines for EBV are relatively new.
What constitutes normal levels of vitamin D is the subject of much debate. Many, if not most, autoimmune diseases are associated with low levels of vitamin D thus raising the possibility of a non-specific effect which may actually be induced by chronic inflammation rather than causing it. Vitamin B12 levels are similarly affected. Clinical trials of vitamin D treatment in MS have given variable results.
Finally the target population for a prospective clinical trial of such therapies is somewhat unclear and may not be practical.
DOES PARKINSON'S DISEASE HAVE A PRION-LIKE PATHOGENESIS: NO
J. Chapman
Department of Neurology, Sheba Medical Center, Tel Aviv University, Israel
Parkinson's disease (PD) is a chronic neurodegenerative process which over a period of many years destroys specific areas of the brainstem, basal ganglia and cortex. A number of proteins are known to accumulate in PD brains including ubiquitine and α-synuclein. Creutzfeldt-Jakob disease is a rapidly progressive disease of all the CNS associated with a self propagating pathological prion which is an abnormal form of PrPC, a normal protein widely distributed in the CNS. It has recently been found that α-synuclein may behave similarly to the prion precursor and propagate between cells.
There are also some clinical similarities between PD and CJD, especially involvement of the basal ganglia and brain stem. However, there are a number of issues that argue caution before PD is declared a prion disorder:
It is not absolutely clear that α-synuclein directly causes propagation of its abnormal forms. Indeed, in the classical PrP associated prion diseases the exact mechanism of propagation has not been defined and it is not certain that these processes are significantly similar. The clinical course of CJD is much faster and more widespread than PD arguing for a more aggressive molecular mechanism. Disease modifying treatments have been found for PD and some may even influence disease progression while no such therapy is available for CJD.
Much work is therefore needed before parallels between CJD and PD become significant.
INFECTIOUS AGENTS: NOT THE CAUSE OF ALZHEIMER'S DISEASE
A. Chaudhuri
UK
Alzheimer’s disease (AD) is the most common cause of dementia in adults. Ad has an age-related increase in the disease prevalence which doubles approximately every 5 years for those between the age of 65 and 85 years. Genetic factors contribute significantly to the risk of developing AD. The age of onset in familial AD is earlier (usually between 45-65 years of age) which remains constant within the kinship affected by the same gene mutation. Apo E genotype ε4 has been implicated in early and late-onset, sporadic as well as familial cases of AD.
Several environmental agents may modify the risk for developing AD. Exposure to toxins (heavy metals such as aluminum and solvents), head trauma and vascular disease have all been proposed to increase the risk of the disease, and few environmental factors such as non-steroidal anti-inflammatory drugs, dietary anti-oxidants and high educational level are considered to be protective. Of these, the vascular pathology appears to play a significant contributory role. However, infection as a cause of AD remains largely speculative and unproven. None of the many transmission studies undertaken in AD has ever been positive. Despite superficial pathological resemblance with prion disease, scrapie- associated fibres characteristic of prion disease have not been seen in AD. In addition, prion protein found in the amyloid of transmissible spongiform encephalopathies is different from the amyloid β protein found in AD.
The cause of AD remains yet unknown but given that age is the single highest risk factor; it is likely that the key mechanism is age-related metabolic changes affecting protein structure in genetically predisposed individuals.
PREVENTING MS WITH EARLY LIFE VITAMIN D SUPPLEMENTATION AND EBV VACCINATION
A. Chaudhuri
UK
Multiple Sclerosis (MS) is a chronic progressive disease and the most common cause of neurological disability in young people in the Western world. The precise etiology of MS is not yet known and the disease pathogenesis is complex, with inflammatory and degenerative changes affecting the white and grey matter of the central nervous system. Present treatment strategies are targeted towards the inflammatory component of the disease and experience suggests that such an approach perhaps slows the rate of progression of disability but does not prevent it in the long term.
Epidemiological studies point to a significant geographical variation in the world-wide prevalence of MS with environmental influence in early life being a key determining factor to the risk of acquiring the disease. Studies are in general agreement that there is an increased risk of MS in the higher latitudes in the northern as well as southern hemispheres, and exposure to sunlight could protect against the risk of disease in monozygotic twins. Low serum vitamin D level has been linked to the first clinical relapse in pediatric patients and vitamin D supplementation in young women has been shown to reduce the risk of MS substantially. There are also several studies which report Epstein Barr Virus (EBV) infection as a risk factor for developing MS in later life. Retrospective serological tests have shown that MS patients have elevated titres of EBV-specific antibody many years before developing first symptoms of the disease. EBV immortalizes B lymphocytes into memory cells which escape immune surveillance and EBV-associated B cell dysregulation has been reported in the brain tissue of MS patients in post -mortem studies.
The association between EBV and MS alone, however, cannot explain the decline in risk among migrants from high to low MS prevalence areas, which support the view that high level of vitamin D is protective, particularly during adolescence. The active form of vitamin D (1,25 dihydroxyvitamin D3) is a hormone with key role in glial and vascular development. It is also an immunoregulatory hormone. Activated T and B-cells express 1,25 dihydroxyvitamin D3 receptors and addition of 1,25 dihydroxyvitamin D3 to cultures of EBV-infected cells inhibited the production of IgM and IgG by the B cells. EBV infection in the setting of relative vitamin D insufficiency in the first two decades of life might well be the specific environmental risk factor which predisposes to the later development of MS. Vitamin D supplementation in early life and EBV vaccination before adolescence would be the most appropriate and effective strategy to minimize the burden of disability imposed by MS in the younger population.
TREATMENT OF BACTERIAL MENINGITIS: LUMBAR PUNCTURE SHOULD BE THE FIRST STEP BEFORE EMPIRIC ANTIBIOTICS
A. Chaudhuri
UK
A definitive diagnosis of bacterial meningitis requires evidence of subarachnoid space inflammation because of bacterial infection. Examination of cerebrospinal fluid (CSF) by lumbar puncture is an undisputable and indispensable part of assessment of patients who present with symptoms and signs of meningitis unless the procedure is contraindicated by reasons of clinical safety. Not only lumbar puncture confirms the diagnosis of bacterial meningitis, CSF microbiology is also very likely to identify the responsible bacterium and confirm its antibiotic sensitivity. CSF formula also reliably distinguishes between acute and subacute or chronic forms of bacterial meningitis. The diagnostic yield of CSF is significantly reduced by prior antibiotic treatment and it becomes virtually impossible to differentiate partially treated acute bacterial meningitis (ABM) from tuberculous meningitis (TBM) by CSF formula when lumbar puncture is delayed.
There are no randomized controlled trials to determine the outcome of ABM based on timing of administration of the antibiotic. There are no large, prospective and controlled studies on the presumed benefit of pre-hospital antibiotic treatment, or the outcome of empiric antibiotic treatment in ABM before diagnostic lumbar puncture is undertaken. No specific symptoms or signs can be regarded as diagnostic of ABM, and a proportion of patients with initial clinical features suggestive of ABM would be expected to have alternative diagnosis, such as viral meningitis, TBM or viral encephalitis. In many large urban hospitals, the major proportion of ABM cases in adults is nosocomial and recurrent episodes of meningitis are frequent. It is often difficult to clinically predict the likely etiological agent for older or immunosuppressed patients with suspected ABM. Antibiotic resistance is increasingly reported in patients even with community acquired ABM caused by pneumococci, and empiric single antibiotic therapy may not cover for resistant organisms. Consequently, empirical antibiotic therapy is likely to delay definitive diagnosis by instilling a false sense of security and in some cases, would be an ineffective and wasteful exercise. In addition, the benefit of high dose dexamethasone therapy would be compromised if empiric antibiotic had to given without steroids before confirming the diagnosis of pneumococcal meningitis.
Available data suggest a cut-off period of 3-6 hours beyond which there is a significant increase in mortality if antibiotic treatment is delayed in ABM. Clearly, there will be situations where the antibiotic treatment may have to be commenced on suspicion before it is possible to confirm the diagnosis of ABM by CSF examination. This could happen in a primary care setting where transfer to a secondary care unit is likely to take some time. Empiric antibiotic treatment should only be initiated for patients with strong suspicion of disseminated meningococcal infection (meningococcemia) because of the unpredictable risk of early circulatory collapse from adrenocortical necrosis. In patients arriving to the hospital, empirical antibiotic treatment for ABM can only be recommended before CSF analysis if lumbar puncture is contraindicated or CSF analysis is likely to be difficult or delayed because of logistic reasons. Ideally, treatment in ABM should be commenced after the diagnosis is confirmed by CSF analysis and antibiotic therapy should follow, rather than precede, lumbar puncture.
CARISBAMATE: ONE OF THE NEW PLAYERS IN EPILEPSY
B. Chmielewska
Department & Clinic of Neurology University of Lublin, Poland
Carisbamate (CBT) is a novel agent in clinical development for adjunctive treatment of epilepsy and neuropathic pain. It possesses potent and broad spectrum activity in a battery of well-characterized animal seizure/epilepsy models and in rodent models of neuropathic pain, essential tremor, mania, depression, and bipolar disorder. Investigations suggest that CBT limits seizure spread, elevates seizure threshold and exerts neuroprotective action. CBT may be useful in treating variety of seizure types, including generalized tonic-clonic and complex partial seizures. Inhibition of voltage-dependent brain-type sodium channel appears to be the major indentified mechanism of its antiepileptic activity. Pharmacokinetics of CBT is linear and characterized by rapid absorption, peak plasma concentration at 0.5-3 hrs, and lack of auto-induction. CBT is metabolized extensively via O-glucuronidation and hydrolysis with following oxidation. It is moderately bound to blood proteins and eliminated via kidneys with elimination T1/2 12 hrs. CBT has minimal effects on CYP2C9-2D6 and-3A4 without any clinically significant effect on VPA, CBZ, or PHT, warfarin and oral contraceptive, but it reduces LTG levels. CBT exposure is reduced by CBZ, PHT and oral contraceptive. Pharmacokinetics of CBT is similar across age groups, sex and race. At of July 2008 36 clinical studies have been completed and 4 open-label studies are ongoing with CBT in a total of 2 979 subject. Safety profile includes mild and transient CNS-related and gastrointestinal adverse events. CBT reduced EEG discharges in subjects with photosensitive epilepsy. As adjunctive treatment CBT significantly reduced seizure rate and elevated responder rate in patients with partial seizures.
ASYMPTOMATIC CAROTID ARTERY DISEASE: PRO-STENT DEBATE
G.S. Chrysant
Peripheral Interventions and Advanced Cardiac Imaging, INTEGRIS Baptist Medical Center & University of Oklahoma, Oklahoma City, USABackground: Since the first report of a successful carotid endarterectomy (CEA) in the Lancet in 1954, CEA has become the most common vascular surgery performed and one of the most common surgeries performed. This is based on several, older clinical trials comparing CEA to medical therapy. While the long term data was very impressive in both symptomatic and asymptomatic individuals (risk reductions of 5-17%), these studies had initial stroke rates at 30 days that were higher than medical therapy. However, the therapy has evolved into a durable and safe procedure generally speaking. What the CEA trials also established was that CEA was safe and durable in the hands of experienced surgeons. In the United States, NASCET trial hospitals, hospitals with large volumes, and large volume operators have excellent results. The same cannot be said for low volume, low operator volume, and non-trial centers. Carotid artery stenting (CAS) evolved due to a need for alternative therapy for patients at high risk for surgical therapy, as well as patients with anatomical contraindications to CEA. What I will show is that CAS data has evolved to the point where it can be considered a safe and durable alternative to CEA in certain populations of patients.
CAS data: SAPPHIRE was the first randomized controlled trial comparing CEA and CAS in high risk patients. The initial trial demonstrated noninferiority of CAS to CEA. There was also a 12% rate of NSTEMI in the CEA population that led to the initial thought that CAS may be better in high risk populations. This result has been proven durable out to two years. Registry data has evolved from early studies where distal embolic protection devices (EPD) were not uniformly used to current data that shows safety in large cohorts of patients. Several centers with experienced physicians have also published data in high risk patients showing safety. While the early CREST data has been favorable comparing CAS with CEA, it has also revealed a population that may be at high risk for revascularization: those patients greater than 80 years of age. Two studies that achieved widespread popular press were EVA 4S and SPACE. These trials showed an early advantage for CEA at 30 days; however, at 2 and 4 years there is no difference in the therapies in terms of adverse events. Both trials have been widely criticized for their design, which included the use or non-use of EPD, as well as multiple EPD and stent designs. Another major problem was the inexperience of the CAS physicians (who could have been performing their first CAS as part of the trial). This is in direct contrast to the other studies mentioned earlier that included physicians with at least 25 procedures as primary operator and one EPD/stent system versus CEA. If I were performing my first CEA as part of a trial, the results may be suboptimal.
Future Directions: Current study data demonstrates that CAS is a safe and durable alternative to CEA in asymptomatic individuals. It has been relatively well established who is at risk for CEA from a medical and anatomical standpoint; however, who is at risk for CAS is less well established. Emerging data and discussion surrounding CAS safety has centered on a few key aspects of the therapy. Plaque characterization has become increasingly utilized as a risk stratifier. Echolucent plaque with a necrotic core as imaged with CTA or IVUS is linked to higher stroke rates in CAS. Patients with poor cerebral reserve are also at risk. The anatomical considerations concern mainly the aortic arch type. Diseased type III arches requiring extensive catheter manipulation are not ideal for CAS and may be a root cause of the higher than expected rates of contralateral stroke with CAS (14% of strokes in CAPTURE 2 and EXACT). These issues can be resolved with preprocedural imaging and with multidisciplinary teamwork; the best therapy can be given to patients with carotid disease. Octogenarians are a different story with a very mixed picture that is too complex for the current discussion.
THE EARLIER THE BETTER: TREATMENT SHOULD NOT BE STARTED IMMEDIATELY ON DIAGNOSIS
C.E. Clarke
University of Birmingham, UK
There are eight reasons why we should not, at present, change the current policy of deferring treatment in Parkinson's disease until functional disability develops:
1 Can we trust the recent rasagiline delayed start design trials? In the TEMPO trial, the results with the 1 mg dose were not significant. In the ADAGIO trial, the results with the 2 mg dose were not significant. The apparent positive results with long-term follow-up in the TEMPO study were compromised by the small number of patients remaining in the study at 6 years. There is also the possibility of selection bias in all delayed start trials, with a tendency to recruit milder slowly progressive patients who can tolerate no treatment for 18 months, so the results may not be generalisable.
2 The beneficial effect on total UPDRS score is not clinically significant: The positive results of both the TEMPO and ADAGIO trials were based on differences below 2 total UPDRS units. It has recently been shown that a clinically meaningful difference in total UPDRS in early Parkinson's disease is at least 6 units.
3 It is not just rasagiline but all symptomatic therapies: Trials with selegiline (DATATOP), levodopa (ELLDOPA) and possibly dopamine agonists (PROUD), show the same benefit in total UPDRS in favor of early treatment
4 Is this effect neuroprotective or non-specific? This small benefit could be due to symptomatic non-specific effects on the musculoskeletal system rather than a true neuroprotective effect (i.e. ‘keeps them supple’). Regulators (EMEA and FDA) require evidence of an effect on a biomarker of disease progression as well as a clinical effect before giving a license for neuroprotection can be issued.
5 No evidence of effect on patient-rated quality of life: There is a suggestion from the PD LIFE audit that quality of life deteriorates quickly in early untreated PD. However, the PD LIFE result may be due to selection bias and such a decline in quality of life has not been found in a similar ongoing Aberdeen study.
6 No evidence that earlier treatment is cost effective: Treating patients on diagnosis would increase the net cost of treatment. There is no evidence, at present, that this is a cost-effective policy change.
7 Adverse effects of earlier treatment: Treating patients earlier may result in more of the standard dopaminergic adverse reactions (i.e. nausea, vomiting, hypotension), along with impulse control disorders (e.g. pathological gambling, hypersexuality, punding). Earlier treatment may also expedite motor complications (i.e. dyskinesias and motor fluctuations).
8 How much evidence must accumulate before we change practice? We have been here before with selegiline. In the 1980s, a large proportion of Parkinson's disease patients were on selegiline in the hope that it was neuroprotective. However, prescriptions fell dramatically in the UK after a report of increased mortality with selegiline (UK PDRG trial). So, we should be more skeptical about large policy shifts based on inconclusive evidence. We must learn from the mistakes of the past!
In conclusion, we must consider whether enough evidence has accumulated for us to change practice. If the existing data is not enough to persuade us to change practice, then we need to perform further trials. These need to be long-term (around 5 years), use quality of life outcome measures (e.g. PDQ 39 and EuroQol EQ 5D) and health economics evaluations (cost utility and cost effectiveness), and include novel biomarkers of disease progression (if these can be found).
BE CAREFUL OF CAA AND MICROBLEEDS WHEN GIVING ANTI-AGGREGANTS AND TPA: CON
C. Cordonnier
France
Brain microbleeds (BMB) are small, rounded, homogeneous, foci lesions seen as hyposignal on T2*-gradient echo MR sequences. They are increasingly recognized neuroimaging findings, occurring in association with cerebrovascular disease, dementia, and normal aging. The prevalence of BMB is around 5% among healthy people, 34% among people with ischemic stroke and 60% among people with haemorrhagic strokes. They can be located in the whole brain parenchyma. There are biomarkers of the severity of the microangiopathy.
Recent studies have established possible connections between normal aging and asymptomatic stages of age-associated small vessel diseases such as hypertensive vasculopathy and cerebral amyloid angiopathy (CAA). They also raised the interesting possibility that isolated lobar BMB may often reflect the presence of advanced CAA.
CAA is characterized by accumulations of amyloid substance in the vessel wall leading to degeneration of smooth muscle cells, with the result that vessels are more susceptible to ruptures and haemorrhages. In CAA, the use of antiplatelet drugs and anticoagulants has been found to be related to increase occurrence of symptomatic haemorrhage.
As markers of underlying haemorrhage-prone vascular pathology, BMB might be expected to predict future risk of symptomatic intracerebral haemorrhage (ICH). The potential association between BMB and risk of future symptomatic ICH raises the question of whether the increased risk might be great enough to tip the risk-benefit calculation away from anticoagulation in situations such as prevention of thromboembolism in individuals with atrial fibrillation, from thrombolytic therapy in ischaemic strokes or even from antiplatelet drugs in primary vascular prevention.
Data suggesting that clinicians should not decide to prescribe or not antithrombotic treatments based on the presence of BMB will be reviewed in this debate.
CITICOLINE
E. Díez-Tejedor
Department of Neurology, University Hospital La Paz, Universidad Autónoma de Madrid
Choline precursors promote repair and growth of cell membranes and hold promise in ischemic stroke. Citicoline is a precursor of phosphatidylcholine (PtdCho) biosynthesis, and an increase in PtdCho biosynthesis prevents apoptosis of neurons and protects against neuronal damage. In addition, citicoline maintains cardiolipin and sphingomyelin levels, stimulates glutathione biosynthesis, activates glutathione reductase, and reduces lipid peroxidation, prevents apoptosis and delay disease progression in the brains of rats with chronic ischemia. Therefore, citicoline conferred acute brainprotection and enhanced plasticity and repair.
Three leading mechanisms have been suggested to mediate the neuroprotective effect observed when citicoline is administered within the first hours of onset of cerebral ischemia: 1) direct repair of neuronal membranes, 2) reduced generation of free fatty acids, and 3) synthesis of membrane phospholipids.
As a protective and repair agent, citicoline has the potential to complement recanalization therapies. Delivered before achievement of recanalization by pharmacologic fibrinolysis or endovascular device, citicoline could enable more threatened tissue to survive until revascularization relieves ischemic stress. Administered in the early period after recanalization, this agent could attenuate reperfusion injury. In the poststroke period, citicoline could enhance the repair and restoration function provided by tissues preserved by reperfusion. In several animal stroke model studies, coadministration of citicoline and fibrinolytics increased the reduction in infarct size attained by the fibrinolytic alone. Particularly, citicoline combined with rtPA seems to be superior to either therapy alone, being the treatment more effective when citicoline was administered after thrombolysis. But probably, citicoline at a high dosage provides an effective neuroprotection and greater benefits, than thrombolytic therapy without the associated risk of hemorrhage.
Similarly, preclinical studies suggest that citicoline can complement other neuroprotective drugs to more fully stabilize the ischemic penumbra or block reperfusion injury. By inhibiting a wider array of molecular pathways that elaborate cell injury in hypoxic environments, combinations of neuroprotective drugs have the potential to be more effective than single agents. In rodent stroke models, administration of citicoline has potentiated the reduction in infarct size achieved by excitotoxity inhibitors (MK-801), presynaptic sodium channel inhibitors (lamotrigine), and calcium channel blockers (nimodipine). Citicoline may also add to the benefits of other agents with neuroplasticity-enhancing effects: citicoline coadministration with a nerve growth factor (fibroblast growth factor) yielded greater benefits than either agent alone.
Citicoline has been studied worldwide in both ischemic and hemorrhagic clinical stroke with excellent safety and possibly efficacy found in several trials.
A total of 13 randomized clinical trials of citicoline in acute stroke have been reported in the literature. Three of these trials reported positive results but did not present global outcome data details permitting amalgamation with other studies. Formal meta-analysis could be undertaken of the remaining 10 trials, enrolling a total of 2279 patients, assignment to citicoline rather than placebo was associated with a substantial reduction in the frequency of death or disability at long-term follow-up (57.0% vs 67.5%, OR 0.64, 95% CI, 0.54-0.77; P < 0.00001).
An analysis confined to the 4 largest (N >100) ischemic stroke patient trials yields a homogenous group of well-reported trials, and finds a smaller, but still highly significant, treatment effect: choline precursors 574/1048 (54.8%) versus placebo 500/773 (64.7%) (OR 0.70, 95% CI, 0.58-0.85; P=0 .0003). In a safety analysis, data across all trials reporting mortality outcomes on deaths by end of trial follow-up show no adverse effect of citicoline: citicoline 179/1235 (14.5%) versus placebo 135/966 (14.0%) (OR 0.99, 95% CI, 0.77-1.21; P= 0. .94). This study-level analysis has advantages of comprehensiveness and of following standard meta-analysis guidelines, but cannot take into account individual patient variability.
By pooling individual patient level data from across the 4 US trials, including only those patients with baseline characteristics rendering them likely to be able to demonstrate a response to an efficacious agent: NIHSS (OR 1,28 (0,99- 1,65), P= 0,057); BI (OR 1,29 (1,03- 1,62), P= 0,028; mRS (OR 1,42 (1,08- 1,88), P= 0,013). The results of these meta -analyses require prospective confirmation in subsequent large trials.
The International Citicoline Trial on acute Stroke (ICTUS) was launched in November 2006 to accomplish this goal. ICTUS is a multicenter trial currently under way in Spain, Portugal, and Germany, and is assigning 2600 acute ischemic stroke patients within 24 hours of onset to high-dose citicoline (2000 g daily) or placebo, with treatment started intravenously for the first 3 days and continued orally until 6 weeks (at moment, there is 1133 patients included). There are certainly aspects of the ICTUS design with which one may quibble. The use of binary extreme excellent outcomes to compose the primary endpoint is moren appropriate for an early recanalization agent than for a protective/ repair agent and may lessen study power.
At the moment, with the preclinical and clinical data available, mainly from meta-analysis, and if, of corse, the ICTUS study shows possitive results, we could consider that citicoline is a good therapeutic option for acute ischemic stroke treatment.
PREGABALIN (AS AN ADD-ON THERAPY IN PARTIAL EPILEPTIC SEIZURES AND ITS INFLUENCE ON ASSOCIATED SYMPTOMS)
V. Donath
Clinic of Neurology, F.D. Roosevelt University Hospital, Banská Bystrica, Slovak Republic
Pregabalin was awarded a licence as an adjunctive anticonvulsant for the partial seizures with or without secondary generalisation in the European Union in 2004 and in the United States in 2003. Pregabalin is recommended also for treatment of neuropathic pain and generalised anxiety disorders.
Pharmacological effect of pregabalin is believed to be the result of the bind to the α-2-δ subunit of P-, Q- and N-type of voltage dependent calcium channels. This results in allosteric channel modulation and decrease of depolarisation induced by calcium influx into the nerve terminals. Result is the decrease of release of excitant neurotransmitters such as glutamate. Even if pregabalin is by the structure similar to gabapentin, the affinity of pregabalin to the α-2-δ modulation place is bigger than with gabapentin, which explains why pregabalin is 3- to 6- times more effective than gabapentin in animal models of seizures.
As pregabalin is practically completely eliminated by renal excretion and it has no impact on the activity of drug enzyme metabolism, no clinically significant drug interactions are expected either. Clinical studies have also shown that pregabalin does not influence the plasmatic concentration of concomitantly administered drugs such as carbamazepine, phenytoin, phenobarbital, valproic acid, gabapentin, lamotrigine, topiramate, oral contraceptives, oral hypoglycemics, diuretics and insulin. Recent studies have shown that enzyme-inducing agents such as carbamazepine may cause reduction in plasma pregabalin concentrations at steady state.
The most frequent adverse events with patients treated with pregabalin are effects from the central nervous system. These effects are only of moderate to medium intensity. These are predominantly dizziness, somnolence, ataxia, asthenia, weight gain, visual impairment, concentration disorders, tremor and peripheral edema.
Four randomized placebo-controlled studies have demonstrated that pregabalin is effective as an adjunct therapy in the treatment of adults with partial epileptic seizures, with or without secondary generalisation. In short-term trials pregabalin significantly reduced the seizure frequency at doses between 150 and 600 mg/day depending on the dose amount and rapid onset of action. In long-term open-label studies almost 8% of pregabalin-treated patients were free from seizures during the last 6 months of observation and almost 4% were seizure-free during the previous 12 months.
In our interventional, prospective, multicentre, open label study the data from 256 patients with epilepsy treated with pregabalin 150- 600mg/day were collected at baseline, week 4, week 12 and after 6 month. Pregabalin as the first add-on therapy in the study performed on our sample of patients with partial seizures with or without secondary generalisation appeared to be an effective antiepileptic drug with important and statistically significant increase in responder rate after 6 months. Apart from the effective treatment of epilepsy, a beneficial effect on associated symptoms, such as anxiety, depressions and sleep disturbances was documented. According to the Qolie 10 questionnaire, most patients experienced an improvement in quality of their lives. Most patients and physicians reported improvements in PGIC and CGIC. Adverse effects of pregabalin were similar to those observed in other double-blind placebo-controlled studies over the last decade. There was no significant weight gain observed with monitored patients. In this way, pregabalin can be recommended as an add-on treatment of patients with partial seizures with or without secondary generalisation.
Pregabalin in add-on therapy offers a good efficacy, tolerance and compliance with partial epileptic seizures. Moreover, this therapy shows a positive effect in the therapy of frequent associated effects. Pregabalin represents an appropriate (advantageous) possibility of add-on therapy of partial epilepsy associated with different comorbidities.
Based on available data of clinical experience adjunctive pregabalin will be a useful therapeutic tool for clinicians, encompassing a long-term treatment of patients with refractory partial epilepsy. The fact that pregabalin is effective in the treatment of generalized anxiety disorder as well as neuropathic pain results in the possibility of treating the patients also with these comorbidities. Pregabalin has not been found to be useful in the therapy of generalised epilepsies and may even aggravate epileptic seizures when used by patients with certain generalized epilepsy syndromes such as progressive myoclonic epilepsies. Among patients with partial epilepsy, no specific factors have been identified that may be used to predict responsiveness to pregabalin. In most cases, an already effective starting dose can be 150 mg/day, given in 2 divided administrations, without the need for titration. If needed, the dose may be increased to 300 and 600 mg/day, with a flexible increase based on the efficacy and tolerability to ensure an optimal clinical response.
AFFIRMATIVE/YES: AD: ARE WE INTERVENING TOO LATE?
V.-O. Emery
Dartmouth Medical School, Department of Psychiatry, Lebanon, New Hampshire, USA
The position taken in this debate is that Alzheimer disease (AD) intervention occurs too late. Consistent with debate protocol, first there will be a brief defining of the terms: Alzheimer disease, intervention, time at intervention. Argument will proceed using the method of representational paradigm. Data from two specific AD populations will be presented to be applied to the broader universe of AD patients. An AD intervention timeline or diachronic continuum is conceptualized. Neuroimaging, neuropathological, psychometric, epidemiological and clinical diagnostic evidence will be discussed demonstrating that AD intervention occurs too late: earlier intervention would work better and is now possible because of improved diagnostic tools and acumen. Earlier intervention would result in the prolonging of normative quality of life for the AD patient and in the significant reduction of social and economic costs to society.
RISK BENEFIT OF NEUROLOGICAL MEDICINES
P. Feldschreiber
UK
This paper will discuss the general principles and specific problems inherent in the evaluation of the risk: benefit of neurological medicines. The evaluation of risk: benefit from data on safety and efficacy from pre- clinical toxicology and pharmacology, clinical trials and post marketing authorization safety surveillance of neurologically and psycho- active drugs poses particular problems of therapeutic acceptability in long term usage. These problems may be exacerbated by the advent of new biologically generated molecules such as monoclonal antibodies, cytokines, new prostanoids and respective specific inhibitor, biological transmembrane receptor agonists and antagonists. This new generation of therapeutic modalities additionally highlights the need for the adoption of common, harmonized guidelines for the regulatory evaluation of risk: benefit of these drugs. Such evaluation needs to take into account both the scientific objective evidence based view of the regulator and the assessment of the lay patient community view of what may be acceptable risks. The history of the initial authorization, subsequent withdrawal and reintroduction of Tysabri (natalizumab) is instructive in this regard. This humanized monoclonal antibody was given accelerated approval for the treatment of relapsing MS with a data base of 5000 patients in clinical trial. Some three months after marketing authorization had been granted 3 patients developed progressive multifocal leukodystrophy and the license was suspended. However such had been the drug’s evidence of efficacy that MS patient groups lobbied successfully to have the marketing authorization reintroduced by the FDA. The paper will discuss this precedent and review the ways in which patient representation can be used in regulatory decision making.
CONTROVERSIES IN TRANSLATIONAL MEDICINE: PATIENTS’ SELECTIONS AND PRECLINICAL MODELS CONGRUENCY IN STROKE DRUG DISCOVERY AND DEVELOPMENT
G. Feuerstein
Discovery Translational Medicine, Wyeth Research, Collegeville PA, USA
Development of neuroprotective agents for treatment of acute focal ischemic stroke has so far proven a most challenging task. In fact, none of the studies tried neuroprotective agents in phase III clinical trials have shown efficacy in meeting expectations by of regulatory agencies in the Western countries. The reasons for this disappointing outcome have been subject of much analysis and debates. One key reason suggested by experts point to the high variability of neurological scales used for patients assessment, in part due to the heterogeneity of the patients in reference to location, time, severity and confounding variables in this patent population. In respect to neuroprotective agents, the fundamental premise in hoping to provide medical benefits by such agents is that the drug protects brain cells (neurons) by blocking the detrimental effects of substances that are released in the ischemic tissue and act in the detriment of neuronal survival. This hypothesis extends the notion that if neuroprotective agents were to provide benefits, a “penumbra” (compromised brain region surrounding the core infarct) must exists for neuroprotective agents to be effective. Thus, trials that test neuroprotective agents should recruit patients that have a demonstrable penumbra. Under this premise, studies aimed to develop methodology to evaluate the penumbra in clinical setting (starting at 6 hrs posit stroke, repeated at 24hrs and extending to 30 days) of acute stroke have been established via a consortium at TMRC. Furthermore, efforts to established congruency between pre-clinical and clinical assessment using BOLD MRI has also been commissioned. Furthermore, development of new motor functional recovery end-points using robotics technology is explored to assess early drug induced recovery in patients. This talk will bring up for discussion the role of the penumbra in identifying patients more likely to respond to neuroprotective agents and possible new biomarkers that provide rapid, consistent and early signals of compound efficacy.
THE SURGICAL MANAGEMENT OF INTRACRANIAL ANEURYSMS: CLIPPING
S. Florian, D. Tusnea, Z. Andrasoni, C. Popa
Neurosurgical Department of the University of Medicine and Pharmacy “Iuliu Hatieganu” Cluj Napoca & Neurosurgical Department of Cluj-Napoca County Emergency Hospital, Cluj-Napoca, Romania
Background: The management of a patient with a cerebral aneurysm is complex, and two accepted treatment modalities are now available. The superiority of the treatment options has not been defined yet, but data are now available regarding the safety and efficacy of each modality. ” An honest and rigorous evaluation by the surgeons and interventionists of their own results with the population they treated at their own institution would certainly be a great first step to clarify the algorithm for treatment, which will always be variable at each institution”.
Objectives: We designed a retrospective study on a single surgeon series of 388 patients harboring solitaire or multiple aneurysms out of 483 operated aneurysms, admitted in our service in a 13-years period, who underwent open-surgery for ruptured or unruptured anterior and posterior circulation aneurysms. The main purpose of the present study is to add some arguments concerning the safety and efficacy of microsurgical clipping performed by a well trained neurosurgical team.
Methods: The authors retrospectively evaluate the indications, the results, the outcome, and the specific characteristics of 483 microsurgically clipped aneurysms, operated by the main author between 1997 and 2008. The data are analyzed in terms of safety and efficacy with special remarks on unruptured aneurysm, where the surgical related complications are evident as are the surgical results. For ruptured aneurysms, the surgical outcome is analyzed in direct correlation with the neurological state at the admission evaluated on Hunt &Hess Scale. Long term follow up ranged from six month to 9 years, with the large majority of cases lost at the follow-up after a two years’ period.
Results: In a 13-years period 388 patients were admitted in our service for intracranial aneurysms, 346 of them being admitted with documented clinical and imagistic findings of intracranial hemorrhage. The rest of 42 patients were admitted for unruptured aneurysms (symptomatic or incidental). 34 patients of our series were harboring multiple aneurysms, among them 63% with 2 aneurysms, the highest number of aneurysms in two patients being 6. One third of our patients with intracranial hemorrhage were admitted within the first 24 hours after the aneurysm’s rupture. Their clinical state was evaluated using Hunt & Hess Scale. In almost all of the cases (98%) definitive clipping was achieved. Considering the location 44% were located at the level of Anterior Communicating Artery (ACoA) Complex (168 cases representing 34, 7%), followed by Middle Cerebral Artery bifurcation (94 cases representing 19%). Seven of our operated aneurysms were located on the posterior circulation.
Discussion: In a surgical perspective the most difficult locations are the ACoA complex and the MCA bifurcation. Considering the multiple aneurysms, our strategy is to clip all the aneurysms in a single stage, using the same unilateral approach, in order to prevent rupture of an unclipped aneurysm. Related to the timing of surgery we are in favor of early surgery, despite of all intraoperative difficulties. Large majority of cases admitted in the first day after the ruptures were operated in an interval of 24-48 hours, except the cases with large intracerebral hematomas, cases that underwent surgery in emergency. Concerning the multiple aneurysms, we prefer to delay surgery 3 to 4 days in order to obtain cerebral relaxation. In terms of safety, when considering the unruptured aneurysms, except one case with giant paraclinoidal aneurysm who was severely disabled(GOS 3) related to surgery (2 %), all the other cases were discharged with GOS 4 and 5 (98%). Our results are close to those reported in the International Study of Unruptured Intracranial Aneurysms (ISUIA) [2] related to mortality (2, 3%) or those reported by Britz and co-workers (5, 5%) [3]. regarding the morbidity, our results are under the median of the both previously mentioned publications (12% in ISIUA report and 8, 5% in Britz report). Concerning the ruptured aneurisms the surgical results were evaluated using Glasgow Outcome Scale at discharge, and we compared these to the clinical findings at admission (Hunt & Hess Grade). At patients admitted with Hunt & Hess grade I and II (91 cases), the mortality in our series was 8, 7%. Obviously the mortality dramatically decreases when taking into consideration three consecutive periods of our personal experience from 12% to 4% in the last period. Comparing our results with those reported in the ISAT study, our results are better even the reported results of the endovascular treated group. The patients with Hunt & Hess grade V underwent early surgery in as many cases as possible with the following results: 61% of the patients survived, from which 36% had good results. Considering that with conservative treatment most of these cases and especially those in Hunt &Hess grade V had a 100% worst prognosis, these results are the best argumentation in favor of early surgery
Conclusions: In surgical management of aneurysm the outcome depends on pre-operative clinical state of the patients, their age, the localization and the morphology of the aneurysm, unruptured associated aneurysms and associated diseases. Nevertheless the surgical expertise is a prominent factor influencing not only the surgical results but even the quality of life of the patient after the operation. Early surgery is recommended in every case when you have departmental and regional requirements, yet positive attitude and experienced surgical skills are mandatory. Early surgery is not recommended for multiple aneurysms in a single stage procedure, giant aneurisms, poor medical condition, posterior circulation aneurysms and beginners in vascular pathology.
WHY SO FEW DRUGS ON THE MARKET FOR AD THERAPY? IS THE TARGET THE PROBLEM OR THE CLINICAL TRIALS
E. Giacobini
Department of Rehabilitation and Geriatrics, University of Geneva Medical School, Geneva, Switzerland
The year 2008 marked the set back of three major clinical trials directed to prevent aggregation or accumulation of beta-amyloid by means anti-aggregants, gamma-secretase modulation or passive immunization.
Preventive therapy has been equally negative.
Why do so many drugs for AD have failed in development? Do we need to rethink the amyloid hypothesis, change the approach and the target, or rethink the trials? We propose that the realities of AD, especially the progression of neuropathology prior to the onset of clinical symptoms require to privilege early targets such as oligomers or early tau and their biomarkers and distinguish signs capable of differentiating individual at risk as surrogate targets for preventive treatment , testing and use in clinical trials.
References: Ezio Giacobini and Robert.E.Becker. One hundred years after the discovery of Alzheimer’s disease. A turning point for therapy? Journal of Alzheimer Disease 12 (2007) 37-52; Robert .E.Becker, Nigel H.Greig and Ezio Giacobini; Why so many drugs for Alzheimer' Disease fail in development? Time for new methods and new practices? Journal of Alzheimer’s Disease 15 (2008) 303-325.
ALBUMIN FOR NEUROPROTECTION IN ACUTE ISCHEMIC STROKE
M. Ginsberg
University of Miami Miller School of Medicine, Miami, Florida, USA
The use of high-dose human albumin as a neuroprotective agent began with serendipitous observations in the author’s laboratory and was pursued in systematic investigations in rodent ischemia models. Moderate- to high- dose human albumin proved to be highly neuroprotective in models of both temporary and permanent focal cerebral ischemia as well as in global cerebral ischemia and traumatic brain injury. In focal ischemia, albumin diminished total infarct volume by two-thirds and reduced brain edema by three-quarters or more, with a therapeutic window of full efficacy extending to four hours [1]. In a meta-analysis of focal ischemia data, albumin-treated rats showed ~80% reductions in mean cortical infarct volume.
Albumin is thought to protect by virtue of its multiple salutary actions. Albumin is the plasma’s major endogenous antioxidant, and at least three mechanisms are contributory: a) its reactive cysteine-34 thiol moiety; b) its ability to bind redox-active transition metals, in particular copper ion; and c) its binding of amphipathic species such as fatty acids and heme, which may participate in injurious redox reactions. Next, albumin maintains plasma colloid oncotic pressure and, in pharmacological doses, induces hemodilution. Albumin also exerts multiple salutary actions on vascular endothelium, decreasing red blood cell sedimentation under low-flow conditions. Albumin reacts with nitric oxide to form a stable S-nitrosothiol that has endothelium-derived relaxing factor-like properties. Albumin also plays a crucial role in the binding and transport of fatty acids. We have shown that albumin reduces ischemic brain swelling; improves blood flow to critically perfused brain regions; reduces postischemic thrombosis and blood-element adhesion within the brain’s microvasculature; improves microvascular flow velocity within the ischemic cortex [2,3]; and supplies important free fatty acids to the postischemic brain.
During 2001-2005, we conducted a National Institutes of Health (NIH)-funded Albumin in Acute Stroke (ALIAS) Pilot Clinical Trial in 82 patients at two clinical sites [4, 5]. This dose-escalation safety trial treated acute ischemic-stroke subjects within 16 hours of stroke onset with 25% human albumin (ALB) in doses ranging from 0.37 to 2.05 g/kg. One-half of the subjects also received standard-of-care i.v. tPA therapy. At 3 months, the NIH Stroke Scale (NIHSS), modified Rankin Scale (mRS), and Barthel Index were measured. Albumin therapy was well tolerated; the sole ALB-related adverse event was mild or moderate pulmonary edema in 13.4% of subjects, which was readily managed with diuretics. The 3- month outcome data were analyzed for suggestions of efficacy by comparing the highest three (putatively therapeutic) ALB dose-tiers (1.37-2.05 g/kg) to the lowest three (presumed sub-therapeutic) doses (0.34-1.03 g/kg), and to historical cohort data derived from the NINDS rt-PA Stroke Study. After adjusting for the tPA effect, the probability of good outcome (defined as mRS 0-1 or NIHSS 0-1 at 3 months) at the highest three ALB doses was 81% greater than in the lower dose-tiers (relative benefit, 1.81; 95% CI, 1.11-2.94); and was 95% greater than in the comparable NINDS rt-PA Stroke Study cohort (relative benefit, 1.95; 95% CI, 1.47-2.57) [5]. The tPA-treated subjects who received higher- dose ALB were 3 times more likely to achieve a good outcome than subjects receiving lower-dose ALB, suggesting a possible synergistic effect of ALB with tPA.
Proceeding from this experience, we received NIH funding to conduct a large phase III multicenter trial designed to test the therapeutic efficacy of ALB in acute ischemic stroke. The ALIAS Multicenter Trial is a randomized, double-blind, placebo-controlled clinical trial whose primary aim is to ascertain whether high-dose ALB therapy administered within 5 hours of stroke onset confers benefit when compared to placebo. The primary efficacy end-point is either an NIHSS score of 0 or 1, or a mRS score of 0 or 1, or both, at 3 months. As initially designed, this trial consisted of two separate but concurrently implemented trials of 900 subjects each: a cohort that received standard-of-care thrombolytic therapy, and a cohort that did not receive tPA. Within each cohort, subjects were randomized in a 1:1 ratio to either 25% human albumin (2.0 g/kg i.v.) or saline placebo. In Dec. 2007 after 434 subjects had been enrolled at 62 North American clinical sites, the trial’s Data and Safety Management Board (DSMB) suspended subject recruitment in order to review a safety concern. In response, the ALIAS principals conducted semi-unblinded safety analyses and submitted to the DSMB a proposal for improving safety, consisting of protocol revisions and a comprehensive site training plan. The study was then approved go forward as a separate trial – “the ALIAS Part 2 Trial” – requiring 1,100 additional subjects. In addition to the previous cardiovascular exclusion criteria, the ALIAS Part 2 Trial now excludes subjects older than 83 years; requires that baseline serum troponin level be normal; and mandates that intravenous fluids are restricted to 4200 ml or less in the first 48 hours and that a diuretic be administered between 12 and 24h. Enrollment in ALIAS Part 2 began in February, 2009. As of August, 2009, 86 subjects have been entered.
References: [1] Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD: Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke 32: 553-560, 2001.
[2] Nimmagadda A, Park H-P, Prado R, Ginsberg MD: Albumin therapy improves local vascular dynamics in a rat model of primary microvascular thrombosis: a two-photon laser-scanning microscopy study. Stroke 39: 198-204, 2008.
[3] Park H-P, Nimmagadda A, DeFazio RA, Busto R, Prado R, Ginsberg MD: Albumin therapy augments the effect of thrombolysis on local vascular dynamics in a rat model of arteriolar thrombosis: a two- photon laser-scanning microscopy study. Stroke 39: 1556-1562, 2008. [4] Ginsberg MD, Hill MD, Palesch YY, Ryckborst KJ, Tamariz D: The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. I. Physiological responses and safety results. Stroke 37: 2100-2106, 2006. [5] Palesch YY, Hill MD, Ryckborst KJ, Tamariz D, Ginsberg MD: The ALIAS Pilot Trial: a dose-escalation and safety study of albumin therapy for acute ischemic stroke. II. Neurological outcome and efficacy-analysis. Stroke 37: 2107-2114, 2006.
ANIMAL STUDIES DO NOT REALLY FAIL US
M. Ginsberg
University of Miami Miller School of Medicine, Miami, Florida, USA
The central issue of this debate is precisely how we should proceed in order to identify new treatments for stroke. The debate’s title, “Why do animal models fail us in finding new treatments for stroke?” is inherently deceptive on two counts: (1) it falsely implies that animal models, by themselves, should be able to predict clinically successful stroke treatments; and (2) it tends to absolve clinical investigations themselves of any responsibility in this process. I assert that animal studies do not, in fact, fail us; and that complex pre-clinical and clinical factors must be considered if one is to obtain a balanced understanding of this area.
• Stated succinctly: there is simply no suitable alternative to animal studies for investigating neuroprotection in stroke. Computer simulations will never suffice. In vitro models (e.g., cell cultures, organotypic slices) lack circulation and thus do not fully resemble stroke-related injury. Clinical intuition (“I think it works”) is fallacious.
• It is unreasonable to assume that animal studies will ever be infallible predictors of clinical success in neuroprotection.
• It is, however, reasonable to expect that animal studies – if scrupulously and intelligently designed and executed – will establish proof-of-principle of neuroprotective efficacy.
• Not all animal studies, however, are equally trustworthy. It is crucial to have tight control of physiological variables; make use of robust, objective outcome measures (e.g., neurological or functional rating scales; quantitative histopathology); employ rigorous statistical design; and observe proper blinding and randomization procedures. Positive studies must be replicated.
• Small mammals (e.g., rodents) are genetically highly homologous to humans (>95%); primates even more so (98-99%). Thus, signaling pathways and injury-mechanisms identified in animals are likely to have human relevance.
• Even so, it is extremely difficult for animal studies to incorporate all the contextual elements shared in common with the human condition (e.g., old age; presence of co-morbid conditions such as hypertension, diabetes, obesity, cigarette smoking, and concomitant medications).
• In addition, human stroke is characterized by great variability in size, location, extent, and even mechanism. Because of this, animal studies will never be infallible, and human clinical trials will always be needed.
• The pre-clinical STAIR (Stroke Academic and Industrial Roundtable) criteria have been “over-hyped” and their intent seriously misconstrued. These criteria were intended merely as guidelines to improve the quality of pre-clinical research, NOT as necessarily reliable predictors of successful translation to the clinic.
• One cannot lay the blame on “failed animal studies” when clinical trials of neuroprotection themselves have, for the most part, been seriously flawed in all or some of the following respects:
o Selection of agents that showed only weak or inconsistent benefit in pre-clinical studies
o Selection of agents based on fallacious mechanistic considerations
o Use of human dosing far below that shown to be efficacious in animal models
o Use of treatment time-windows grossly longer than the effective therapeutic window demonstrated in animal studies
o Choice of poor functional outcome measures
o Inadequate statistical design
o Choice of agents for clinical trials based primarily on corporate, market-driven decision-making rather than on the basis of hard, objective science. This has led to the wasted expenditure of millions of dollars or Euros on poorly conceived clinical trials in the false hope of “hitting a home run” – i.e., demonstrating a clinical benefit (no matter how small or trivial) that would allow a propriety product to be approved and marketed for profit.
Examples will be used to illustrate these points.
GENETICS OF MIGRAINE: IMPLICATIONS FOR NOVEL TREATMENTS
L. Griffiths, R. Lea, N. Colson, S. Quinlan, J. MacMillan
Genomics Research Centre, School of Medical Science, Griffith University, Gold Coast, Queensland, Australia, The Institute of Environmental Science and Research Ltd. Wellington, New Zealand and Queensland Clinical Genetics Service, Royal Children's Hospital Health Service District, Brisbane, Queensland, Australia
Migraine is a severe neurological disorder that affects a significant proportion of the population. Prevalence estimates for the disorder vary between 12 and 25% depending on the population studied. The disorder has a significant genetic component showing high levels of familial aggregation. Although a number of genes involved in a rare and severe sub-type of migraine, termed familial hemiplegic migraine have been identified, the number and identity of all the genes involved in the more common types of migraine have yet to be defined. Genetic characterization of migraine have implicated genomic regions on a number of chromosomes, including 1q, 4q, 5q, 6p, 11q, 14q, 15q, 17p, 18q, 19p and Xq. In addition, candidate gene association studies have identified several susceptibility variants that are implicated in the disorder. Neurotransmitter pathways have been the main focus of studies investigating the molecular mechanisms of the disorder. However vascular and hormonal triggers disturbances also occur in migraineurs, as highlighted by alterations in cerebral blood flow and hormonal triggers of migraine, particularly in women and hence factors affecting these functions may also is involved. This presentation will focus on migraine gene studies in our laboratory, including recent studies implicating dopamine beta hydroxylase and MTHFR functional variants, as well as presenting an overview of results from a recently completed clinical trial that involved genetic profiling in conjunction with a nutriceutical therapeutic treatment. These clinical trial results are very promising and highlight the potential importance of pharmacogenetic interventions in this disorder.
VITAMIN SUPPLEMENTATION AS A TREATMENT FOR MIGRAINE
L. Griffiths, R. Lea, N. Colson, S. Quinlan, J. MacMillan
Genomics Research Centre, School of Medical Science, Griffith University, Gold Coast, Queensland, Australia, The Institute of Environmental Science and Research Ltd. Wellington, New Zealand and Queensland Clinical Genetics Service, Royal Children's Hospital Health Service District, Brisbane, Queensland, Australia
Migraine is a common, debilitating neurological disorder that imposes a severe burden on the health and well-being of sufferers. At least 12% of the Caucasian populations suffer from migraine with current treatments exhibiting variable efficacy. Studies in our laboratory have been directed towards identifying the genes that play a role in migraine. These studies have implicated a number of genes including the methylene tetrahydrofolate reductase (MTHFR) gene. Specifically, a mutation in this gene has been shown to occur more often and potentially play a role in migraine susceptibility, particularly in those suffering from migraine with aura (MA). The mutation in this gene (C677T) has been shown to reduce the activity of the MTHFR enzyme and lead to increased levels of homocysteine. Numerous studies have shown that plasma homocysteine levels are significantly higher in patients with coronary artery disease, vascular disease, and stroke. Migraine is also associated with an increased risk of stroke, particularly in sufferers of MA. The MTHFR risk genotype results in increased levels of circulating homocysteine and it has previously been shown that additional dietary folate and increased vitamin B levels can have an important effect reducing these levels. Since the MTHFR mutation has been implicated in migraine susceptibility, it is possible that appropriate dietary additions directed towards this mutation could influence migraine onset and severity. To address this possibility we recently undertook a 6 month double-blind placebo controlled trial to determine the effects of folate-vitamin B supplementation on migraine frequency, severity and disability.
The results of this trial were very promising. 52 individuals completed the trial with results showing a significant reduction in migraine symptoms in those on treatment. As expected vitamin supplementation reduced homocysteine levels, with a 39% decrease (~4 μmol/L) compared to baseline, a reduction not seen in those on placebo (P = 0.001). More significantly though, vitamin supplementation reduced the prevalence of migraine disability from 60% at baseline to 30% after 6 months (P=0.01), with no reduction in the placebo group (P>0.1) and headache frequency and pain severity were reduced (P<0.05), with no reduction in the placebo group (P>0.1). Also in this patient group the treatment effect on both homocysteine levels and migraine disability was associated specifically with MTHFR C677T genotype (P<0.05). This trial utilised a relatively inexpensive, non-toxic vitamin therapy, and if shown to be effective in subsequent studies, has the potential to make a significant contribution to the health and quality of life of many migraineurs. We are now undertaking a larger trial to further investigate this vitamin therapy as an inexpensive and effective prophylactic option for the treatment of migraine.
SHOULD VALPROATE BE PRESCRIBED TO WOMEN OF CHILDBEARING AGE WITH IDIOPATHIC GENERALIZED EPILEPSY (IGE)? THE "NO SIDE"
A. Guekht
Russian State Medical University, Moscow, Russia
Treatment of IGE: there is a choice of other effective antiepileptic drugs (AEDs): IGE represent various syndromes that include multiple seizure types - absence, myoclonic, and generalized tonic–clonic (GTC). Depending on the age at onset and predominant seizure type, individual syndromes of IGE are defined. However, when the treatment options of IGE are discussed, it is important to remember that most clinical trials address treatment of seizure types not syndromes (Panayiotopoulos, 2005; Bergey, 2005, Hitiris, Brodie, 2005).
In general, IGE well respond to treatment, with 80–90% becoming fully controlled. For many years valproate (VPA) was considered the first line treatment; now the new drugs like lamotrigine (LTG), levetiracetam (LEV) and topiramate (TPM) are increasingly used in the treatment of IGE (Bergey, 2005; Montouris G., Abou-Khalil.C, 2008; Curatolo et al., 2009). There is an evidence-based support for the efficacy of LEV in myoclonic seizures of juvenile myoclonic epilepsy (JME), LTG in primary generalized absence, and TPM in primary GTCS. LTG, TPM, LEV and zonisamide (ZNS) have shown evidence of efficacy in JME. The choice of the specific AED should take into consideration the most prominent seizure type and co-morbidities. For example, LEV could be the preferred choice if myoclonic seizures are the dominant seizure type, LTG – in cases of absence seizures. The presence of obesity could favor choosing TPM or ZNS, migraine - TPM (Prasad et al., 2003; Coppola et al., 2004; Faught, 2004; Sullivan & Dlugos, 2004; Bergey, 2005, Montouris, Abou-Khalil, 2009).
Valproate in the treatment of women of childbearing age with IGE: important concerns: Both the disease and the use of AEDs present women of childbearing age with many problems and can affect the menstrual cycle and other aspects of reproductive endocrine function, contraception, fertility, pregnancy, bone health (Crawford, 2005). Cosmetic issues should also be considered.
Long-term VPA treatment has been associated with reproductive endocrine disorders characterized by hyperandrogenism and polycystic ovaries (Isojarvi et al., 1993, 2001; Murialdo et al., 1997, 1998; Betts et al., 2003; Morrell et al., 2003). The effect is probably drug specific as these findings have also been noted in women treated with VPA for bipolar disorders (O’Donovan et al., 2002; McIntyre et al., 2003) as well as in animal studies (Roste et al., 2001, 2002; Tauboll et al., 2003; 2009; Nelson-DeGrave et al., 2004). It was shown that VPA affects steroidogenesis in follicular ovarian cells by increasing testosterone and decreasing estradiol secretion (Gregoraszczuk et al., 2000; Tauboll et al., 2002, 2003).
The most frequently cited cosmetic issues with VPA are weight gain and hair loss; the occurrence of weight gain is not consistently dose- related and cannot be predicted.
There are two major concerns regarding the treatment of women of childbearing age with VPA - the risk of major congenital malformations (MCMs) and cognitive consequences for offspring.
Several large prospective pregnancy registries demonstrated a consistent pattern of increased risk for major congenital malformations (MCMs) with VPA use as monotherapy, compared to nonexposed populations and to other AEDs in monotherapy (and more pronounced if VPA was included in polytherapy).
The UK Pregnancy Register (Morrow et al., 2006) reported that polytherapy combinations containing VPA carried a higher risk of MCMs than those without VPA (OR 2.49; 95% CI 1.31–4.70). For monotherapy, the MCM rate was greater for pregnancies exposed to VPA (6.2%; 95% CI 4.6–8.2%) than to CBZ (2.2%; 95% CI 1.4–3.4%; OR 2.78 (p < 0.001). The data from the North American AED Pregnancy Registry (Wyszynski et al., 2005) showed that with first-trimester VPA monotherapy exposures, major birth defects occurred in 10.7% (95% CI 6.3– 16.9%) of infants, as compared with 2.8% in infants exposed to other AED monotherapies. Therefore, there was a fourfold increased risk for having an offspring with a major birth defect for VPA-exposed women compared with those taking other AEDs. Meador et al. (2006) demonstrated increased risk of VPA exposure compared to other monotherapies as a combined group (CBZ, LTG, and PHT) with an RR of 4.59 (95% CI 2.07–10.18%). Many studies have supported a dose- relationship for VPA, with an increased risk for MCMs with VPA doses above approximately 1,000 mg/day (Mawer et al., 2002; Artama et al., 2005). However, for patients with IGE in North American Registry mean±SD daily VPA doses were close to this “borderline” values (920 ± 477 mg). In fact, no threshold dose at which risk significantly increased could be identified, although there was a strong trend toward lower risk at <750 mg/day (5.5% versus 15.1% for doses >750mg/day, p = 0.063) (Bromfield et al., 2008).
Several studies confirmed that intrauterine exposure to VPA reduces cognitive outcome (Adab et al., 2001; 2004; Gaily, 2004). For instance, a retrospective survey in the UK demonstrated an especially high risk of VPA for the neurodevelopment of children exposed in utero (Adab et al., 2001). Compared to children of women with epilepsy on no AEDs, the ORs for additional educational needs were 1.49 for all children exposed to AEDs and 3.4 for children exposed to VPA monotherapy. A recently published prospective multicenter study in the USA and the UK (Meador et al., 2009) demonstrated that in utero exposure to VPA, as compared with other AEDs, is associated with an increased risk of impaired cognitive function at 3 years of age.
Conclusion: Concerns regarding the use of VPA in women of childbearing age with IGE were expressed in many studies (Crawford, 2005; Wyszynski et al., 2005; Morrow et al., 2006; Bromfield et al., 2009, Pennel, 2009 and many others). As underlined by Meador et al., (2009) “valproate should not be used as a first-choice drug in women of childbearing potential”. This notion is consistent with the conclusion of the Report of the AAN and AES (2009) with the Level B recommendation for avoidance of VPA and AED polytherapy during the first trimester of pregnancy in order to decrease the risk of MCM and throughout pregnancy in order to prevent reduced cognitive outcomes.
However: The choice of AED should always be a mutual decision of the doctor and the woman of childbearing potential with IGE, based on the discussion of the risks and benefits of all the appropriate AEDs available.
It is reasonable to recommend one of the new AEDs as initial treatment of IGE (while also considering limitations and risks of new AEDs - for instance, pharmacokinetic alterations of LTG) (Tomson, Battino, 2007; Pennell, 2008).
Further prospective data from EURAP as well as from other large pregnancy registries are essential;
Randomized trials comparing VPA and the new AEDs as first-line therapy in IGE are needed.
ASYMPTOMATIC CAROTID STENOSIS: INTERVENTION OR
JUST STICK TO MEDICAL THERAPY: THE DEFENSE
A. Halliday
Surgical Sciences, St George’s University, London
Patients with significant carotid artery stenosis (60-99%) are 3 times more likely to suffer disabling or fatal ischemic stroke when compared with the general population.1 Approximately 100,000 people in the UK and at least one million people in Europe have severe carotid stenosis.2,3 Trials in symptomatic patients support use of CEA to prevent stroke, but there is currently no robust evidence that allows us to predict which asymptomatic lesions will cause a stroke; therefore, for some patients with tight asymptomatic carotid artery stenosis intervention may be indicated.4,5
Carotid stenosis may be treated surgically by open removal of plaque through carotid endarterectomy (CEA) or by endovascular means, using carotid stenting (CAS); these procedures may actually cause stroke if thrombosis occurs in the endarterectomised vessel or if emboli are dislodged from the carotid plaque and block the distal cerebral circulation. The long-term outcome balancing peri-operative risk and stroke prevention benefit for both procedures needs careful examination and good medical treatment is essential to minimize stroke risk from other factors.
The use of prophylactic carotid endarterectomy in patients with asymptomatic carotid stenosis has caused considerable controversy. The prevalence of carotid stenosis >50% is 5e10% in the older population, and their annual stroke risk in screening studies has been estimated as 1e3%. However, even with this lower risk, because most strokes arise in patients without previous symptoms, the potential benefit of intervention in the form of CEA in asymptomatic patients may be important. A number of randomized trials have been conducted recently comparing stroke rates in asymptomatic patients. The first Asymptomatic Carotid Surgery Trial (ACST-1) and the Asymptomatic Carotid Atherosclerosis Study (ACAS) enrolled almost 5000 patients with carotid stenosis and no stroke or stroke-like symptoms in the preceding 6 months. In ACST-1, which reported 5-year results in 2004, 3120 patients were randomized between immediate CEA and medical therapy, and delayed CEA (waiting until operation more clearly needed, for example, if symptoms occurred) and medical therapy. Results showed that even though immediate CEA involved a small (~3%) but definite peri-procedural risk of stroke or death, there was a significant (~3% versus ~12%, p < 0.001) reduction in the subsequent stroke rate over the next 5-years and a net gain (~6% versus ~12%) in the overall 5-year risk of stroke or peri-procedural death.9 In 1995, for a smaller group, ACAS had shown similar results and CEA reduced the overall 5-year risk of ipsilateral stroke and death from 11% to 5.1% (p<0.004).10 These results provided robust and contemporary evidence that CEA prevents stroke in patients with asymptomatic carotid stenosis.
Recent data arising from the SPARCL trial indicates that the increased use of statins does reduce stroke risk in patients with asymptomatic carotid disease (although their degree of stenosis was undefined).11 From ACST we know that CEA in patients up to 75 years also significantly reduces stroke risk, when intervention for these asymptomatic patients is indicated. CEA is a well proven procedure but less invasive endovascular stenting is now an attractive alternative.
Endovascular treatment for carotid stenosis is commonly performed under local anesthetic using remote percutaneous arterial access. Compared to CEA, CAS can be used to reach surgically inaccessible lesions, avoids a surgical wound, reduces the risk of cranial nerve injury, is usually done with shorter hospital stay and might reduce the risk of peri-procedural myocardial infarction or stroke. However, there are complications associated with stenting: injury to the access vessels from introduction of wires and catheters may result in vessel dissection; crossing of the atherosclerotic lesion to place the stent may cause distal embolisation and stroke, even though fine umbrella-like cerebral protection devices have been developed to provide protection. Radiological contrast can precipitate allergic reactions and is nephrotoxic, particularly in patients with previously compromised renal function. CAS is already in wide use: for example, approximately 7000 carotid stents per year are inserted in Germany and worldwide most stents are used in patients with asymptomatic carotid disease. There remains, however, substantial uncertainty concerning the immediate hazards and long-term reliability of CAS, with recent studies highlighting a possible increased incidence of 30-day adverse events in octogenarians and in those with unfavorable plaque morphology.12 A recent systematic review and meta- analysis of ten mainly symptomatic trials of CEA versus CAS concluded that both procedures were equivalent in terms of death and nonfatal myocardial infarction. The pooled data was not sufficient to determine the differential impact on stroke rates, though results suggested a trend towards an increase in stroke risk following CAS.
No large trial has specifically set out to compare CAS and CEA in asymptomatic patients, although four published trials of CEA versus CAS and the lead-in phase of the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) have included some asymptomatic patients15-19. In randomized trials patient populations were mostly symptomatic and none reported separate subgroup analysis for asymptomatic patients. A pooled subgroup analysis of asymptomatic patients from these trials did not provide meaningful conclusions because of small numbers of patients and wide confidence intervals.13 The only trial involving solely asymptomatic patients was small (N=85) and reported no major complications (stroke or death) in either CEA or CAS treated groups.18 Because of the lack of randomized trial evidence, a 2008 review of guidelines by the European Stroke Initiative recommends that CAS in patients with asymptomatic carotid stenosis should only be undertaken in randomized controlled trials. Because stroke risk (for CEA and for CAS) is lower for asymptomatic patients, a large international multi-centered trial is required to compare immediate (30-day) procedural risk and long-term stroke risk reduction in patients with asymptomatic carotid stenosis. ACST-2 aims to compare CEA with CAS in asymptomatic patients and provide clinicians with robust evidence as to which (if either) intervention is least hazardous and which can provide best long-term stroke reduction benefit. The trial is designed to fit in easily with everyday clinical practice. The trial protocol is available through our website ( www.acst.org.uk ), The ACST-2 trial is funded by the UK National Institute of Health Research (NIHR) Health Technology Assessment program and by the BUPA Foundation. The trial sponsor is the University of Oxford.
References: 1 Taken from: European Stroke Initiative website Major risk factors for stroke, http://www.eusi-stroke.org . 2 Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, editors. Global burden of disease & risk factors Washington DC: Oxford University Press and The World Bank; 2006. 3 Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bam Ford JM, et al. The cost effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review. Health Technol Assess 2002; 6:7. 4 European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet 1991; 337:1235-43. 5 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade stenosis. N Engl J Med 1991; 325:445-53. 6 PROGRESS Collaborative Group. Randomized trial of a perindopril- based blood-pressure e lowering regimen among 6,105 individuals with previous stroke or transient ischemic attack. Lancet 2001; 358:1033-41. 7 Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin- converting-enzyme inhibitor, ramipril, on cardiovascular events in high- risk patients. N Engl J Med 2000; 342:145-53. 8 Heart Protection Study Collaborative Group. Effect of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20,536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004 Mar 6; 363(9411):757-67. 9 MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet 2004; 363:1491-502. 10 Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA 1995 May 10; 273(18):1421-8. 11 Amarenco P, Benavente O, Goldstein LB, Callahan 3rd A, Sillesen H, Hennerici MG, et al. Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke 2009 Apr; 40(4):1405-9. 12 Sayeed S, Stanziale SF, Wholey MH, Makaroun MS. Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J Vasc Surg 2008; 47:81-7.
13 Coward LJ, Featherstone RL, Brown MM. Percutaneous transluminal angioplasty and stenting for carotid artery stenosis (Cochrane review). In: The cochrane library, 1. Chichester, UK: Wiley & Sons; 2009. 14 Murad MH, Flynn DN, Elamin MB, Guyatt GH, Hobson RW, Erwin PJ, et al. Endarterectomy vs. stenting for carotid artery stenosis: a systematic review and meta-analysis. J Vasc Surg 2008; 48:488-93. 15 CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Lancet 2001; 357:1729-37. 16 Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351(15):1493 -501. 17 Ling F, Jiao LQ. Preliminary report of trial of endarterectomy versus stenting for the treatment of carotid atherosclerotic lesions in China (TESCAS-C). Chin J Cerebrovasc Dis 2006; 3(1):4-8. 18 Brooks WH, McClure RR, Jones MR, Coleman TL, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy for treatment of asymptomatic carotid stenosis: a randomized trial in a community hospital. Neurosurgery 2004; 54(2): 318-24. 19 Hobson RW, Howard VJ, Roubin GS, Brott TG, Ferguson RD, Popma JJ, et al. Carotid artery stenting is associated with increased complications in octogenarians: 30- day stroke and death rates in the CREST lead-in phase. J Vasc Surg 2004; 40(6):1106-11.
CIDP CAN BE TREATED PERMANENTLY WITH IVIG
H.-P. Hartung
Department of Neurology, Heinrich-Heine-University Düsseldorf, Germany
Since its first description in the mid-70s, chronic inflammatory polyradiculoneuropathy (CIDP) has been recognized as an important cause of peripheral neuropathy. This progressive or relapsing disease is also believed to be an autoimmune disease.
Currently, corticosteroids and intravenous immunoglobulin (IVIG) are used as first-line treatment options in the management of CIDP. A number of short-term investigations into the efficacy of IVIG in the treatment of this condition suggest that IVIG might reduce the disability caused by CIDP when compared to placebo. However, until recently, there were limited data regarding the long-term efficacy and safety of IVIG in the treatment of CIDP, resulting in IVIG remaining unlicensed for the treatment of this disorder in many countries.
Recently, 10% caprylate-chromatography purified intravenous immune globulin (IVIG-C; Gamunex®, Talecris Biotherapeutics Inc) was compared with placebo to ascertain its short and long-term efficacy and safety in the management of CIDP (ICE study).1 The primary endpoint was the percentage of patients achieving and maintaining an improvement of one or more inflammatory neuropathy cause and treatment (INCAT) points through to week 24.
A total of 117 adult patients were enrolled in this randomized, double-blind, placebo-controlled, response-conditional crossover trial. IVIG-C or placebo was administered every three weeks for up to 24 weeks in the initial treatment period. Those patients who improved were randomly reassigned in a blinded 24-week extension phase.
The percentage of patients achieving the primary outcome was significantly increased in the IVIG-C group in comparison to the placebo group (P=0.0002). In addition, patients in the extension phase receiving IVIG-C experienced a longer time to relapse when compared to patients receiving placebo (P=0.011). The incidence of serious adverse events per infusion was 0.8% and 1.9% for IVIG-C and placebo-treated patients respectively.
These data suggest that IGIV-C is safe and effective for patients with CIDP up to 48 weeks and holds out the hope that efficacy may be sustained for an even greater period of time.
References: 1. Hughes RA et al. (2008) Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomized placebo-controlled trial. Lancet Neurol 7: 136– 144.
ORAL FINGOLIMOD
H.-P. Hartung
Department of Neurology, Heinrich-Heine-University Düsseldorf, Germany
Oral fingolimod (FTY720) – a sphingosine 1-phosphate receptor modulator1 – is the lead compound in a novel class of drugs for the treatment of multiple sclerosis.2 The efficacy, safety and tolerability of oral fingolimod are currently being assessed in a large worldwide phase III clinical trial program in MS with more than 4000 patients.
Sphingosine 1-phosphate receptors are widely expressed on a variety of cells including lymphocytes, as well as on neural cells.These receptors play a key role in the normal egress of lymphocytes from the lymph nodes. 6 Fingolimod reduces the number of activated lymphocytes circulating in the bloodstream, but a proportion of memory and effector lymphocytes remain in the circulation and are available to migrate to peripheral tissues.
Oral fingolimod significantly reduced both the annualized relapse rate and inflammatory activity on MRI scans at 6 and up to 48 months compared with placebo in patients with relapsing MS. It was generally well tolerated up to 48 months of treatment. There was no evidence for effects on heart rate with chronic therapy, pulmonary function and mean arterial blood pressure remained stable during the extension phase. A risk minimization plan is under development for later use of fingolimod.
References: 1. Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002;277(24):21453-21457. 2. Adachi K, Chiba K. FTY720 Story. Its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immunomodulators based on reverse pharmacology. Perspectives in Medicinal Chemistry 2007;1:11–23. 3. Baumruker T, Billich A, Brinkmann V. FTY720, an immunomodulatory sphingolipid mimetic: translation of a novel mechanism into clinical benefit in multiple sclerosis. Expert Opin Investig Drugs 2007;16(3):283-289. 4. Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 2007;115(1):84-105. 5. Dev KK, Mullershausen F, Mattes H, et al. Brain sphingosine-1- phosphate receptors: implication for FTY720 in the treatment of multiple sclerosis. Pharmacol Ther 2008;117(1):77-93. 6. Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 2005;23:127-159. 7.Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006;355(11):1124-1140. 8. Montalban X, O'Connor P, Antel J. et al. Oral Fingolimod (FTY720) Shows Sustained Low Rates of Clinical and MRI Disease Activity in Patients with Relapsing Multiple Sclerosis: 4-year Results from a Phase II Extension. In: 19th Meeting of the European Neurological Society (ENS); 2009 20 –24 April; Milan, Italy: Oral Presentation, 2009.
CSF EXAMINATION IS MANDATORY IN EVERY CIS CASE, REGARDLESS OF MRI FINDINGS
E. Havrdova
Czech Republic
Though there is accumulating evidence that B cells are key components in the pathophysiology of multiple sclerosis (MS), and possible therapeutic target as well, extensive work on intrathecally produced antibodies has not yet clarified whether they are pathogenetically relevant in MS. Recently clonally expanded plasma cells and single-cell RT-PCR of correctly paired heavy and light chain immunoglobulin genes revealed myelin reactivity of antibodies produced by CSF plasma cells in immunofluorescence stainings of MS lesion tissue. The occurrence of oligoclonal bands varies significantly according to geographical regions, being 97% in most European countries, 86% in Turkey and 33% in prototypic MS in South-East Asia. In European countries the lack of OCBs in the first demyelinating event means a red flag in differential diagnostic process (ADEM, optic-spinal MS, leukodystrophy, hereditary ataxias, etc.). There have been a lot of speculations on the prognostic value of the presence of OCBs in patients with first symptoms suggestive of MS. The cohorts studied for sufficiently long periods are small and the results differ significantly, again depending on geographical region. The presence of optic-spinal MS in patients of European ancestry, and the continuum of both clinical and laboratory markers stretching over these types of inflammatory demyelization needs rethinking of diagnostic criteria for MS in near future.
FUMARATE
E. Havrdova
Czech Republic
Dimethyl fumarate has shown both anti-inflammatory effect and significant protection of myelin and axonal integrity during the chronic phase of experimental autoimmune encephalomyelitis. There was clear reduction in macrophage infiltration of the spinal cord of treated animals.Activated microglia secrete pro-inflammatory cytokines involved in the breakdown of blood-brain-barrier. By activating the transcription factor nuclear- factor-E2-related factor 2 (Nrf2), fumarates induce expression of endogenous antioxidative factors in rat brain cells in vitro, which may protect the CNS from the detrimental effect of reactive oxygen intermediates released as part of the inflammatory process in multiple sclerosis. Fumarates also inhibit the transcription factor nuclear-factor κB, which is important for the expression of several inflammatory cytokines, chemokines, and adhesion molecules. DMF affects adhesion molecule expression on human leukocytes and their rolling behavior in vivo, indicating that DMF directly affects the initial step of leukocyte extravasation. In the Phase II study with daily doses of 120, 360 or 720mg of BG-12, and placebo, MRI analysis showed a significant and dose-dependent reduction of brain lesion activity with the best results in the highest dose treatment group. BG-12 was generally safe and well tolerated. Common adverse events included flushing, headache and gastrointestinal symptoms, and decreased during the extension phase of the study. The incidence of infections was low and no opportunistic infections occurred. Currently two Phase III studies are testing the clinical effect against placebo (DEFINE Study), and comparator glatiramer acetate (CONFIRM Study).
DOES PD HAVE A PRION-LIKE PATHOGENESIS? YES
R. Hilker
Department of Neurology, Goethe University, Frankfurt/Main, Germany
Prions are infectious protein agents known to cause animal and human brain diseases by transmitting a misfolded protein configuration throughout the brain tissue. Prion protein cellular (PrPc) is the endogenous and physiological form of the molecule which has a predominant α-helix structure and can be found on cell membranes of many tissues. In contrast, the isoform Prion protein Scrapie (PrPSc) is an infectious agent with a much higher proportion of a β-sheet structure and a strong tendency to aggregate in form of amyloid fibers and plaques. It is able to convert normal PrPC protein into the infectious isoform by conformation changes. The aggregation of PrPSc leads to neurodegenerative diseases of the brain with amyloid plaques and subsequent cell death. There are three pathomechanisms in prion diseases: 1. sporadic configuration change from PrPC to PrPSc, 2. genetic origin by mutations in the PRNP gene and 3. transfection of PrPSc and subsequent conversion of PrPc by the ingested pathogen.
Parkinson’s disease (PD) is a neurodegenerative disease with proteinaceous neuronal inclusions (Lewy bodies and Lewy neurites) due to proteolytic stress and the accumulation of misfolded proteins. There are now several lines of evidence for the hypothesis that PD might be a prion disorder and that α-synuclein (SNCA), a key protein in the pathogenesis of PD, behaves like a prion: 1. both prion diseases and PD can manifest as sporadic or genetic disorders. Mutations in the SNCA gene locus are known to cause familial PD. 2. Both prion diseases and PD are characterized by the accumulation of β-sheet protein aggregates (amyloid in prion diseases, Lewy bodies in PD). 3. Both prion diseases and PD show a transformation of the native α-helix into the pathogenetic β-sheet protein configuration of PrPC and SNCA respectively. Protein aggregates are able to promote further conformation changes of native α -helix proteins in terms of a vicious cycle. 4. both prion diseases and PD reveal a kind of transmissibility: while the infectious nature of spongiform encephalopathies (e.g. BSE of Creutzfeldt-Jacob disease) is undoubtedly proven, recent data demonstrated that SNCA can be directly transferred from SNCA overexpressing neurons to adjacent healthy stem cells in transgenic animals with the subsequent development of inclusion bodies and signs of neuronal degeneration (Desplats et al., 2009). Moreover, embryonic dopaminergic transplants in PD patients have been shown to develop SNCA-positive Lewy bodies (Kordower et al., 2008). Both findings suggest that misfolded SNCA is transmitted from affected to healthy neurons inducing the fatal chain reaction of further protein misfolding. According to Braak’s PD staging, the misfolded SNCA might enter the central nervous system via the olfactory epithelium and enteric nerves and reach the olfactory bulb and the dorsal motor nucleus of the vagus in the lower brainstem via antero- and retrograde transport (Braak et al., 2003). Afterwards, the disease process spreads in a sequential manner particularly in neurons which are rich of native SNCA, such as the enthorhinal cortex, hippocampus, amygdala, substantia nigra, insula and temporo-basal cortex. In conclusion, these findings suggest that PD might be a prion disorders resulting from increased production or insufficient degradation of misfolded toxic SNCA. The abnormal conversion of native to β-sheet SNCA is triggered or supported by various factors, such as hereditary factors, aging, oxidative stress or environmental toxins.
References: Braak, H., Rub, U., Gai, W.P., Del Tredici, K., 2003. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110, 517-536. Desplats, P., Lee, H.J., Bae, E.J., Patrick, C., Rockenstein, E., Crews, L., Spencer, B., Masliah, E., Lee, S.J., 2009. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A 106, 13010- 13015. Kordower, J.H., Chu, Y., Hauser, R.A., Freeman, T.B., Olanow, C.W., 2008. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med 14, 504-506.
WHAT IS THE BEST TREATMENT FOR ADVANCED FLUCTUATING PD? APOMORPHINE
R. Hilker
Department of Neurology, Goethe University, Frankfurt/Main, Germany
Apomorphine is the most potent dopamine agonist and comparably effective as levodopa in the treatment of Parkinson’s disease (PD). It binds to striatal D1 and D2 receptors with a very short half-life of nearly 45 minutes. Bye virtue of a distinct hepatic first-pass effect, it has to be given subcutaneously either as single injection via pen or continuously through a portable infusion device (apomorphine pump). The latter has been established as one option of the so-called continuous dopaminergic stimulation (CDS). This concept allows the long-lasting, steady stimulation of striatal dopamine receptors which matches the physiological situation in the brain much closer than the pulsatile overflow of the striatum with dopamine resulting from oral ingestion of levodopa plus carbidopa having a short half-life of around 90 minutes. The undulating property of standard dopamine replacement therapy is associated with the development of motor fluctuations in long-standing PD on the one hand and one major reason for an insufficient symptomatic relief of manifest fluctuations in advanced disease stages on the other. In contrast, CDS is an effective treatment for off-phase symptoms and dopaminergic dyskinesia in suitable PD patients. Continuous infusions of apomorphine subcutaneously or of a levodopa-carbidopa gel via percutaneous endoscopic gastrostomy in the duodenum (duodopa pump) are the two options of CDS providing nearly similar symptom relief as deep brain stimulation of the subthalamic nucleus (STN-DBS). Overall, apomorphine infusions have been shown to improve off-time between 50 and 80%, whereas dyskinesia reduction occurs after a few weeks of treatment by widening of the therapeutic window. The selection of suitable patients to apomorphine, duodopa or STN DBS is a very important step to ensure the optimum treatment for the individual patients. STN DBS promises great benefit but requires the strict consideration of some exclusion criteria due to surgical risks (e.g. intracranial hemorrhage) and stimulation-induced side effects. Of these, an advanced age over 70 years, severe postural instability and cognitive, affective and behavioral deficits, such as dementia, depression, psychosis and impulse control disorders are clinically the most relevant ones. From this reason, many advanced stage PD patients are not suitable candidates for STN DBS and excluded from neurosurgery, but can definitely benefit from CDS as less invasive and straining therapy options. When the decision between apomorphine and duodopa infusion is pending, many advantages argue for the dopamine agonist as the treatment of choice. The apomorphine pump avoids the small, but existent risk of surgical intervention (percutaneous endoscopic gastrostomy) and possible long- term adverse events, such as bacterial stomatitis and peritonitis. The technical requirements on the infusion system are less in apomorphine than in duodopa, where catheter displacement and dysfunction (e.g. occlusion) with subsequent loss of therapeutic efficacy are common adverse events. The apomorphine pump itself is much smaller and lighter than the duodopa system and, therefore, easier to handle for patients and caregivers. It is also related with a clearly less stigma by the external device. Finally, the remarkably lower treatment costs for apomorphine versus duodopa infusions in standard daily dosages should be kept in mind.
LATEST ADVANCES IN THE PATHOPHYSIOLOGY OF MIGRAINE
T. Ho
Merck Research Laboratories
Migraine is a complex, multisymptomatic disorder, influenced by genetic and environmental factors, with clinical manifestations that vary considerably within attacks and across patients. Several neurotransmitters and neuromediators are involved in the pathophysiology of migraine, and migraine is the ultimate result of a complex neural network dysfunction that leads to an over-sensitive brain. During migraine, typical sensations such as visual inputs, sounds, and sensations became bothersome and lead to photophobia, phonophobia and pain. These external inputs are no longer filtered and are transmitted to the brain. Calcitonin Gene Related Peptide (CGRP) appears to play an important role in modulating the excitability of the brain. It is thought that during a migraine, the CGRP level is elevated and this leads to activation of CGRP receptors. The activation of CGRP receptors in turn, either through autocrine or endocrine pathways, modulates the excitability of trigeminal ganglia; trigeminal nucleus caudalis periaqueductal grays, and allows the transmission of these incoming signals to many parts of the brain. CGRP receptor antagonists, such as telcagepant and MK-3207 appear to block the activation of CGRP receptors and inhibit the hyper-excitability of the brain. The role of CGRP in migraine pathophysiology and the effectiveness, safety and tolerability of CGRP receptor antagonists in acute migraine will be reviewed.
IN PATIENTS REHABILITATION: STATE OF ART, PROFIT AND OUTCOME (SOCIAL REINTEGRATION)
V. Homberg
St.Mauritius therapy clinic Meerbuch and Heinrich Heine University Düsseldorf, Germany
Historically rehabilitation medicine in many countries emerged from spa -like activities using locally available „healing“resources such as thermal water, mud etc. Since modern neurological rehabilitation medicine has however been developed into a high quality form of evidence based medicine taking advantage from the enormous progress in the neurosciences.
This raises the question what is the best form of treatment organisation and where such treatment can optimally be delivered. There is certainly consensus that today rehabilitation should be done close to the patients` family, home and workspace. Threfore out-patient services especially in bigger urban areas seem to be a good alternative to the classical inpatient paradigm.
Nevertheless still inpatient hospital based structures form the backbone of neuro-rehabilitation.
This is primarily due to the fact that today (especially in those countries with heavily shortened length of stay in acute care) most patients after CVA or TBI entering rehabilitation are in still critically ill conditions requiring a high standard of medical and nursing care and are not able at this stage to be taken care of at home.
It can be shown that only with a well functioning inpatient rehabilitation system optimal results can be achieved in well structured inter-disciplinary teams allowing for optimal recovery of function, avoiding outlasting impairments and guaranteeing desired chances for the patients' participation in society.
During the course of the rehabilitation process it can be decided when treatment can be switched to outpatient services. It has to be noted that even in Germany with a very developed
Rehabilitation network we are still lacking appropriately designed structures of post rehab after care (primarily outpatient-based) to ascertain outlasting rehabilitation results e.g. by coaching services for patients and care givers.
Therefore there is no true controversy between in- and outpatient rehabilitation but there should rather be a well defined shake- hand.
CONTINUOUS DOPAMINERGIC STIMULATION (CDS) BY TRANSDERMAL LISURIDE IN RESTLESS LEGS SYNDROME (RLS) AND PARKINSON’S DISEASE (PD)
R. Horowski, K.P. Latte, D. Palla, H. Benesh
Axxonis Pharma AG, Berlin & Somni bene Institute for Medical Research and Sleep Medicine Schwerin, Germany
CDS is believed to be an excellent option for longterm therapy of PD (postponing motor complications) and RLS (postponing augmentation). To provide CDS, we have developed a transdermal formulation of the high affinity/high activity dopamine agonist lisuride (lisuride TDS). Compared to oral lisuride with its short tmax and t/2, lisuride TDS reaches peak plasma levels much slower followed by stable levels over ≥ 48 hrs, with a 20 cm² patch releasing about 5 mcg/hr (which with application every other day (e.o.d.) replaces an average of 8 lisuride tablets (à 0.2 mg) over two days). Variability in lisuride plasma levels is only ~ 50 % compared to the tablets and depends mostly on skin properties and liver blood flow but the patch circumvenes GI and first pass liver metabolism. As a consequence, dosing becomes quite easy with no or just 1 up-titration step to 20-40 cm² e.o.d. Early adverse events (AEs) such as nausea, emesis, orthostatism and dizziness which are associated with rapidly increasing plasma levels are rather rare (in PD as add-on even in the placebo range) and this might also affect sedation and even sleep attacks but there is need for further studies. Clinical studies in RLS show also excellent efficacy in the IRLS but also regarding day-time symptoms and in patients with a history of augmentation (in descriptive analysis superior over oral ropinirole). In the responder rate (50 % improvement in IRLS) lisuride effects were does-dependent (PLA 27 %, 10 cm² 43 %, 20 cm² 62 % and 40 cm² 66 %) and better than with oral ropinirole (PLA 25 %, ROP 41 %, LIS 56 %, p = 0.04). No case of augmentation has been reported in the double-blind and longterm studies (1 and 2 years) by investigators or patients, but after 4 years there was one suspect case of gambling. Most frequent AEs were non-allergic reversible application site reactions (mostly erythema, sometimes with pruritus) which also caused a significant number of patients to withdraw from the studies. Additional longterm studies are necessary to evaluate further possible benefits from longterm CDS with transdermal lisuride.
ART AND NEUROSCIENCE: ON EVOLUTIONARY MEANING OF ART AND ITS NEUROBIOLOGICAL RELEVANCE
C. Hoschl, F. Shpaniel
Prague Psychiatric Centre & 3rd Faculty of Medicine, Charles University, Prague
Neurobiological hypothesis of the evolution of art suggests that it was the size of social groups what determined the development of the human frontal cortex. Social cohesion is accomplished by mutual grooming. The cohesion of the groups of hominids larger than 100 individuals could not be secured enough by “face-to-face” grooming up to 20-30% of time available. More time, however, could not be allocated for grooming, because other, life-important activities would be dangerously shortened. Instead, an additional effective way to maintain the social cohesion had developed in forms of vocalizations and drumming, which can be understood as a “social grooming”. Here the direct line to singing and to language can be traced. (In the Czech language, for example, drbat means both to tattle and to tickle). The gene FOXP2, which occurs roughly 500-200 thousand years before present, seems - together with neo-cortex (social brain) and social grooming - to be a precondition to the language development. Music and language employ to some extent identical brain structures. Play (both music and game) activates also mirror cells. Mirror neurons play important role in empathy and in a prediction of behaviour of others. Prediction of behaviour of others is a fundamental precondition of survival and represents a substantial selective advantage. “Useless” child plays, tags, barley-breaks, ding-dongs, prattles, and tattle and gossips of adults as well as “barren” activities like music, singing, drumming, and dancing are altogether operations employing and training the life saving mirror neuronal systems, which are essential for our ability of insight.
Art, sport, game and play have therefore crucial importance for the development of our abilities to empathise and to predict the behaviour of others, to recognize their emotions, to maintain a social cohesion and therefore alliances, and last but not least of our capacity of a self- reflection. Art and game thus represent a common condition for the development of language, “motions” and e-motions. From the evolutionary perspective, as “coaches” of our capacity to predict they represent a substantial selective advantage.
References: Brown S et al. Music and language side by side in the brain: a PET study of the generation of melodies and sentences. European J Neurosci, 23, 2006: 2791–2803. Dunbar RIM. The social brain: mind, language, and society in evolutionary perspective. Annu Rev Antropol 2003; 32:163-181. Iacoboni M et al. Cortical mechanisms of human imitation. Science 286, 1999: 2526–2528. Iacoboni M et al. Grasping the Intentions of Others with One’s Own Mirror Neuron System. PloS Biology 3, 2005:529–535. Kawabata H, Zeki S. Neural Correlates of Beauty. J Neurophysiol. 2004; 91:1699-1705, Koelsch S. Significance of Broca’s area and ventral premotor cortex for music-syntax processing. Cortex 2006; 42:518-520. Lewis P. Musical minds. TRENDS in Cognitive Sciences, 6, 2002; 9: 364-366. Molnar-Szakacs I, Overy K. Music and mirror neurons: from motion to ’e’motion. SCAN, I, 2006: 235–241. Höschl C, Španiel F. Umění a (neuro)věda [in Czech]. Sanquis, 2008;56:46-49.
HERPES SIMPLEX VIRUS TYPE 1, WITH THE TYPE 4 ALLELE OF THE APOLIPOPROTEIN E GENE, CONFERS A MAJOR RISK OF ALZHEIMER’S DISEASE
R. Itzhaki, M.A. Wozniak
Faculty of Life Sciences, University of Manchester, UK
Herpes simplex virus type 1 (HSV1) was first suggested as a possible factor in Alzheimer’s disease (AD) because in the severe but rare brain disorder, herpes simplex encephalitis (HSE), it targets the brain regions primarily damaged in AD. Also, survivors of HSE usually suffer from memory loss and cognitive impairment – typical AD symptoms. HSV1 is a neurotropic virus that infects about 90% of the adult population, a ubiquity consistent with a possible role in AD as the disease is highly prevalent. After initial infection, HSV1 remains life-long in the peripheral nervous system (PNS) in a latent state but can be reactivated by events such as stress and immunosuppression, leading to a productive infection and virus replication, and in some people this results in cold sores. This ability to remain latent – and to reactivate – could explain why AD symptoms become manifest in older people.
Whether HSV1 is present in brain in normal circumstances was previously unknown. Using the ultra-sensitive method of solution polymerase chain reaction (PCR), we discovered that HSV1 DNA resides in latent form in a high proportion of brains of elderly people, including AD patients – in the temporal and frontal cortices. (Later, five other groups broadly substantiated this finding.)
We then found that HSV1 confers a high risk of AD when in brain of APOE-ε4 carriers, this joint genetic-environmental risk factor applying to 60% of our cases. (We showed also that AD brain is not predisposed to HSV1 infection, nor does APOE-ε4 carriage predispose to HSV1 infection.) Consistently, we found that APOE-ε4 is a risk for cold sores. This modulatory effect of APOE on microbial damage has been substantiated by our subsequent discovery that APOE genotype governs outcome of infection in certain diseases of known infectious cause. Further, three groups have demonstrated isoform-specific effects of APOE on viral load, or on viral expression, in HSV1-infected APOE- transgenic mice, APOE-ε4 leading to potentially greater viral damage than did APOE-ε2 or ε3,. This supports our concept that in humans, HSV1 infection is indeed modulated by APOE, with greater viral damage occurring in brain of APOE-ε4 carriers.
Subsequently, by detecting an HSV1-specific intrathecal antibody response, we confirmed HSV1 presence in brain and revealed that it had replicated there, causing a productive, perhaps recurrent, infection, in AD sufferers and elderly controls. Interestingly, we detected HSV1 only rarely in brain of younger people, suggesting that it reaches the brain in older age, perhaps due to the decline in the immune system.
HSV1 causes damage both directly and via inflammatory and oxidative processes, and in turn, inflammation can reactivate latent HSV1. Systemic infections can stimulate immune cells in the brain leading to inflammation, and causes cognitive decline in elderly humans (and memory loss in animals): several studies have shown an association between extent or number of infectious episodes and cognitive decline in the elderly. Thus repeated systemic infection and subsequent inflammation in brain could reactivate latent HSV1 in elderly humans, thereby greatly augmenting the damage, and in APOE-ε4 carriers leading to the development of AD.
We have linked HSV1 directly to Aβ42 (the main component of amyloid plaques) and to abnormally phosphorylated tau (the main component of neurofibrillary tangles). Using immunocytochemistry (ICC) and ELISA with several antibodies to Aβ, we found that HSV1 infection of cultured human neural cells causes a striking increase in intracellular level of Aβ1-42. ICC showed too the presence of oligomeric Aβ in the infected cells. Consistently, the enzymes involved in generation of Aβ from APP – β- and γ-secretases – increased in HSV1-infected neural cells. Also, we found by immunohistochemistry (IHC) an appreciable amount of Aβ1-42 in brain of HSV1-infected mice. Further, using ICC, ELISA and Western blotting, we showed that in the infected cells tau was abnormally hyper-phosphorylated at several sites characteristic of tau in AD brain. The enzymes responsible for such phosphorylation – protein kinase A (PKA) and glycogen synthase kinase 3β (GSK 3β) - increased too. A similar study elsewhere has shown that tau hyperphosphorylation and also altered microtubule dynamics and neurite damage occur in HSV1-infected cortical neurons from foetal mice.
Most excitingly, using a combination of in situ PCR with IHC for Aβ1-42 or thioflavin S staining for amyloid plaques we discovered that HSV1 DNA is located very specifically within amyloid plaques in human frontal and temporal cortex. Ninety percent of the plaques contain HSV1 DNA, and in AD brain, 72% of the viral DNA is associated with plaques (only 24% in elderly normal brains, perhaps reflecting lesser production or greater clearance of amyloid). (We can refute the possibility of artefactual binding due to plaque “stickiness”.)
Our in situ PCR findings, together with the increased deposition of Aβ after infection, strongly support a causal role for HSV1 in formation of toxic amyloid products and plaques. We propose that during events such as stress and peripheral infection, inflammation and oxidative processes occur in brain which reactivate latent HSV1 (as in the PNS) and the virus then causes a productive but localized infection – possibly a “mild”, atypical encephalitis. (Cases of mild and of recurrent HSE have been reported; these perhaps occur relatively often but are under- diagnosed) Infection would cause both direct damage and indirect, inflammatory-mediated damage, and in APOE-ε4 carriers the damage would be greater, eventually leading to AD. The mechanism might well involve viral-induced increases in Aβ and AD-like tau, both of which have been strongly implicated in AD aetiology. HSV1 is uniquely able to cause such features as, unlike other viruses that cause these features, it is present in a high proportion of elderly brains.
All the data strongly suggest that HSV1 has a major role in AD. They are the first to indicate a likely cause of AD, in contrast to almost all other studies which describe a feature or symptom, and are thus the first to indicate a means of inhibiting further deterioration by combating a major cause (rather than combating the symptoms): use of antiviral agents as a treatment to slow AD progression and possibly future use of vaccination against the virus to prevent AD.
References: 1. Itzhaki RF et al. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet, 349, 291-293 (1997) 2 Wozniak MA et al. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer's disease patients J Med Virol 75, 300- 306 (2005). 3. Itzhaki RF, Wozniak MA. Herpes Simplex Virus Type 1 in Alzheimer's disease: The Enemy Within. J Alzheimers Dis. 2008; 13:393 -405. 4. Wozniak MA, Itzhaki RF, et al. Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett. 2007; 429:95-100. 5. Wozniak MA et al. Alzheimer's disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis. 2009; 16:341-50. 6. Wozniak MA et al. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009; 217:131-8.
THE CASE FOR HSV-1 NOT BEING THE CAUSAL AGENT OF IDIOPATHIC BELL’S PALSY
P. Kennedy
Department of Neurology, University of Glasgow, Institute of Neurological Sciences, Glasgow, Scotland, UK
Idiopathic Bell ’s palsy (BP) accounts for approximately two thirds of cases of acute peripheral facial weakness, the remaining cases being caused by a number of different specific conditions such as neuroborreliosis, Ramsay Hunt syndrome due to Varicella-Zoster virus (VZV), amyloid and parotid tumours (1). Although about 70% of patients with BP recover completely even without treatment, the most optimal method of treatment of BP is still a very important issue because of the risk of permanent paralysis, contractures and aberrant reinnervation of the facial nerve in the remaining 30% of patients.
Because BP is known to be associated with oedema and inflammation of the facial nerve, corticosteroid treatment in such patients, particularly in the early stages, was an obvious possibility. While some earlier studies suggested that corticosteroid treatment probably improves outcome, two large recent studies have now established beyond doubt that treatment with prednisolone given within 72hr of the onset significantly improves the prognosis of BP (2, 3). The Swedish study involving 829 patients reported that the time to recovery was significantly shorter in the 416 patients who received prednisolone compared with the 413 patients who did not (2), and 72% of those receiving steroids were fully recovered at 1 year compared with 57% in the group not receiving steroids. The Scottish Bell’s palsy trial involving 551 patients showed that following treatment with prednisolone within 72hr of the onset of BP, 83% of patients were completely recovered at 3 months compared with 63.6% of those who received placebo, while at 9 months the corresponding figures were 94.4% and 81.6% (3). In neither study was there evidence of significant harm from steroids.
Could idiopathic BP be caused by a virus such as Herpes Simplex virus (HSV-1) or VZV? Based on reports that HSV-1 DNA was detected in the endometrial fluid of 11 out of 14 patients with BP, but not in controls or non-idiopathic BP cases (4), and that 8-28% of cases of BP were attributed to ‘zoster sine herpetic’ (5), it seemed that there was a rationale for herpesviruses being the cause of a significant number of BP cases. In this talk I shall argue the case for herpesviruses not being the likely cause of most cases of idiopathic BP.
Several important caveats should be emphasized in attributing a viral cause to a medical condition. First, the detection of a virus in patients’ blood, CSF or other tissues in a disease does not by itself provide evidence of a cause and effect relationship, and this applies particularly to herpesviruses. Both HSV-1 and VZV are present in latent form in up to 90% of spinal and trigeminal ganglia in normal individuals (6), so detection of these viruses in peripheral neural tissues is not necessarily significant. Second, latent herpesviruses may reactivate in response to a variety of triggering stimuli such as trauma and infection, so their presence during BP is not necessarily causal. Third, increased antibody responses to a particular virus may not necessarily mean that the virus is playing a pathogenic role in the condition, but may be a non- specific response resulting from polyclonal activation of memory B cells from some other cause.
The only clinically important and relevant test of whether herpesviruses cause idiopathic BP is the response of patients to anti-viral therapy. Even if HSV or VZV were playing some sort of role in the pathogenesis, this is of no practical clinical importance unless aciclovir, or oral agents such as valaciclovir, are effective treatments for BP. The key evidence against this can be gleaned from several recent studies. The Scottish Bell’s palsy study of 551 patients, which was double-blind, placebo-controlled and randomized, showed that treating patients with BP with aciclovir, either alone, or in combination with prednisolone, produced no additional benefit when compared with patients who had received prednisolone alone. After 9 months, a complete recovery was seen in 94.4% of patients receiving prednisolone alone, 92.7% for aciclovir plus prednisolone, 85.2% for placebo alone and 78% for acyclovir alone. Overall, 85.4% of those receiving aciclovir (with and without prednisolone) recovered fully versus 90.8% not receiving aciclovir (difference not significant but favoring no aciclovir). This indicated that treatment with prednisolone was actually slightly better than prednisolone combined with aciclovir. A similar lack of efficacy of anti- viral therapy was recently reported in the large Swedish study which was also double-blind, placebo-controlled and randomized in 16 centers over 6 years, and showed that there was no difference in time to recovery between the 413 patients treated with valaciclovir (3000mg /day for 7 days which should be effective against both HSV-1 and VZV), and the 416 patients who did not receive valaciclovir. At 12 months, recovery was seen in 71% of the patients receiving prednisolone plus placebo, 57% receiving placebo plus placebo, 58% of those receiving valaciclovir plus placebo, and 74% receiving prednisolone plus valaciclovir. Further, one earlier smaller study from Japan showed that in 150 patients with BP, there was no significant difference in the recovery rates between patients who had been treated with prednisolone alone or valacicovir plus prednisolone (7). However, the findings of these 3 studies conflict with that of Hato et al from Japan which reported that a combination of valaciclovir (1000mg/day for 5 days) and prednisolone was more effective than prednisolone alone, with the combined regime producing complete recovery in 96.5% of 114 patients compared with 89.7% of 107 patients receiving prednisolone and placebo. Further, 90.1% of cases with complete facial palsy receiving valaciclovir and prednisolone recovered fully compared with only 75% of such severe cases receiving prednisolone and placebo(8). The reasons for this discrepancy are unexplained, but as has been pointed out by others, this latter study has several methodological flaws, including the fact that both treatment administration and BP outcome assessment were carried out by investigators who were aware of the study-group assignments, only 75% of patients enrolled in the study underwent randomization, and patients comprising the 25% drop out rate were excluded from the analyses of results (1, 3).
Whether valaciclovir may possibly have a role in treating severe cases of BP will need to be addressed in future studies, but the weight of evidence to date shows that there is not a convincing case for a causative role of HSV-1 in most cases of idiopathic BP.
References: 1) Gilden, DH, Tyler, KL (2007). N.Engl.J.Med, 357:1653-55. 2) Engstrom, M, Berg, T, Stjernquist-Desatnik A et al (2008). Lancet Neurology. 7:993-1000). 3) Sullivan, FM, Swan, IRC, Donnan, PT (2007), N.Engl.J.Med, 357:1598-607. 4) Murakami, S, Miizobuchi, M, Nakashiro, Y et al (1996), Ann.Intern.Med, 124; 27-30 5) Hato, N, Murakami, S, Gyo, K, (2008), Lancet, 371:1818-20. 6) Bradley, BM, Bloom DC, Cohrs RC, (2003), J.NeuroVirol, 9:194-204. 7) Kawaguchi K, Inamura, H, Abe Y et al (2007), Laryngoscope, 117:147- 56. 8) Hato, N, Yamada H, Kohno H et al (2007), Otol.Neurotol, 28:408-13
RUFINAMIDE
V. Komarek
Prague Epilepsy Center Motol, Czech Republic
Rufinamide, a triazole derivative, reduces the recovery capacity of neuronal sodium channels after inactivation, limiting neuronal sodium- dependent action potential firing and mediating anticonvulsant effects.
In experimental models, oral rufinamide suppressed pentylenetetrazol-induced seizures in mice (ED50 45.8 mg/kg) but not rats, and was active against MES-induced tonic seizures in mice (ED50 23.9 mg/kg) and rats (ED50 6.1 mg/kg). Intraperitoneal rufinamide suppressed pentylenetetrazol-, bicuculline-, and picrotoxin-induced clonus in mice (ED50 54.0, 50.5, and 76.3 mg/kg, respectively). Rufinamide was partially effective in the mouse strychnine test. The behavioral toxicity of rufinamide was similar to or better than established AEDs tested in this study. In general, the protective index of rufinamide was greater than that of the other AEDs (White, 2008).
In human, adjunctive oral rufinamide was more effective than placebo in treating seizures associated with treatment-resistant Lennox- Gastaut syndrome in a randomized; double-blind trial involving 138 adult and pediatric patients (Kluger, 2007). Compared with placebo, rufinamide 45 mg/kg/day resulted in significantly superior reductions in drop attacks (median change −42.5% vs +1.4% with placebo) and total seizures (−32.1% vs −11.7% with placebo). Furthermore, these beneficial effects of rufinamide on seizure frequency were maintained throughout a subsequent 3-year, open-label extension study. Relative to placebo, adjunctive rufinamide significantly reduced the frequency of partial seizures per 28 days in adult patients with inadequately controlled partial seizures in two well designed trials, with a higher proportion of rufinamide recipients achieving a reduction of ≥50% in partial seizure frequency per 28 days (Deeks, 2006).
The common adverse events (reported by 10% of patients receiving rufinamide) were somnolence (24.3% with rufinamide vs 12.5% with placebo) and vomiting (21.6% vs 6.3%).
Rufinamide was granted orphan drug status by the European Medicines Agency (EMEA) and the US Food and Drug Administration (FDA) in 2005. Rufinamide was approved by EMEA as an adjunctive treatment for seizures associated with Lennox-Gastaut Syndrome (LGS) in children and adults.
EBM IS OF MARGINAL HELP FOR THE COMMON CLINICAL PRACTICE IN NEUROLOGY
A.D. Korczyn
Israel
Evidence-based medicine, or EBM, is becoming accepted as the gold standard regarding clinical decisions, particularly therapy. It has been developed as a reaction to the great amount of accumulating research and frequently conflicting conclusions. It is needed in an area of budgetary constraints and is aimed at a guide to clinicians in making decisions, but is also used by administrators and health providers when deciding about approval of therapies.
Basically EBM conclusions are based on meta-analysis of available studies. However, one basic question in a meta-analysis is whether the data included consist of all the available data or at least are representative of the full data set.
The only sources of information on which EBM conclusions are based are full articles describing randomized controlled trials (RCT's) published in English. RCT's are expensive and complicated experiments, which are initiated and planned almost exclusively by the pharmaceutical industry. In order to maximize their chances of reaching positive results, the protocols have sepcific inclusion and exclusion criteria. These include age, comorbid conditions and administration of several other drugs. The conclusions, therefore, have limited generalizability. In addition the duration of the studies is limited and therefore data on delayed adverse events are not included.
More importantly, the sponsors also have enormous influence on the publication of the results. Typically they choose the authors, provide them with digested data and a draft protocol, and decide on whether the manuscript will be published, when and where. Coupled with editors' preference for positive news, this means that when RCT's arrive at results which are "negative," these are less likely to be published (or otherwise destined for submission to a local, non-English journal which will not be included in the EBM analysis).
As a result of these limitations, data on several drugs are not available. These include older drugs, for which the patient right have expired, as well as of situations with a low commercial interest, e.g., orphan diseases, rare symptoms of common diseases, as well as common symptoms of common diseases which are not specific, such as depression in neurodegenerative diseases.
Another important consideration is that the indication for drugs is based on the definition of the disease in question. This is itself problematic, since there is sometimes little consensus on the definitions. For example, there is no accepted definition of vascular dementia, the definition of Alzheimer's disease is largely made by exclusion of other disorders; and that of Parkinson's disease or multiple sclerosis is constantly changing. How can we trust that results of studies done on a patient population defined in one way will be applicable to a group defined differently?
When a new drug is developed and marketed, the relevant question for the clinician is not whether the new drug is effective, but rather how does it compare to the best drug already available in the market. This information is not explored by the industry, and thus we do not know whether memantine is actually superior to its close chemical relation, amantadline, in dementia, whether rasagiline is any better than selegiline in Parkinson's disease, and whether duloxetine is superior to fluoxetine (or indeed amitriptyline).
Therefore EBM data fail to answer the relevant questions:
• Can results be extrapolated outside the study population (in terms of age, comorbidity, duration, or geographical area)?
• They do not provide comparative data for previously-used drugs in terms of efficacy, safety and price.
IMPLANT FOR AUGMENTATION OF CEREBRAL BLOOD FLOW TRIAL-24 (IMPACT-24). A PIVOTAL STUDY EVALUATING THE EFFECTIVENESS AND SAFETY OF THE ISCHEMIC STROKE SYSTEM (ISS*) FOR TREATMENT OF ACUTE ISCHEMIC STROKE
D. Kreiger
Sweden
Background: The Sphenopalatine Ganglion (SPG) is a source of vasodilating parasympathetic innervation to the anterior cerebral circulation. In rat stroke models, stimulation of the SPG, results in augmented cerebral blood flow, reduced infarct volume and improved neurological outcome. A clinical pilot study (ImpACT-1), evaluated the ISS for the treatment of acute ischemic stroke (AIS) in the anterior circulation within 24 hours of stroke onset. This study demonstrated a safe and effective profile and promoted the initiation of a clinical pivotal study (ImpACT-24). Design: A multinational, randomized, double blind, parallel arm; sham control (2:1), adjunctive to Standard of Care study. 600 patients with AIS in the anterior circulation, baseline NIHSS 7-18 and ability to initiate treatment 8-24hrs from stroke onset will be enrolled. The ISS is implanted, by a trained implanter, adjacent to the SPG through the greater palatine canal using a minimal invasive approach. Patients are randomized in a 2:1 ratio to either ISS Stimulation or Sham Stimulation. ISS/sham stimulation is applied 4 hours daily for 5 consecutive days, after which the implant is removed. Outcome Measurement is effectiveness measured by mRS and NIHSS at 90 days. Safety outcome measurements are reflected by incidence of adverse events. A Data Safety Monitoring Board is reviewing ongoing safety data. Results: To date, 45 patients have been enrolled. Mean age 70.5 yrs, mean treatment time from stroke onset 17.3 hrs, Median baseline NIHSS 12 (range 7-18). Conclusion: ImpACT-24 is an ongoing clinical trial based on extensive preclinical studies and a clinical pilot study. It is designed to assess the safety and effectiveness of the ISS for treatment of AIS in a prolonged therapeutic time window than currently available and is enrolling patients 8-24 hours after stroke onset.
* The ISS is an investigational device limited for investigational use; for the ImPACT-24 study group.
MCI IS NOT A USEFUL CONCEPT IN PD
J. Kulisevsky
Movement Disorders Unit, Neurology Department, Saint Pau Hospital and Ciberned, Saint Pau Research Institute, Autonomous University of Barcelona, Spain
Cognitive impairment (CI) is a well-recognized feature of Parkinson’s disease (PD) and may be present from the earliest stages of the disease. Early PD is usually associated with subtle CI without clinically apparent cognitive decline, with deficits being uncovered following accurate neuropsychological examination.
In non-demented PD (PD-ND) patients, neuropsychological examinations reveal a dysexecutive syndrome in nearly all subjects that occurs early in the course of the disease. Up to half of these patients may also experience visuospatial defects and free-recall memory problems. Non-demented PD patients can also show additional impairments in predominantly cortical-based cognitive functions. An estimated 15–20% of PD-ND patients' exhibit errors in confrontation naming tasks, and others develop amnestic disturbances characterized by encoding deficits rather than impaired retrieval. Such findings are typically reported in patients with Alzheimer’s disease (AD) and are a reflection of the dysfunction of the temporal lobe. Alterations in neuropsychological tasks suggestive of neocortical dysfunction become increasingly evident during the transition from PD-ND to PD with dementia (PDD), with more severe and frequent defects in confrontation naming and semantic – rather than phonemic – verbal fluency.
In a recent community-based longitudinal study of newly diagnosed PD patients, picture copying (a task sensitive to the functionality of the posterior cortical visual areas) and semantic verbal fluency were found to be the most significant neuropsychological predictors of cognitive decline leading to dementia. Thus, PD-associated CI seems to be characterized by a predominantly attentional-dysexecutive impairment accompanied by additional deficits in tasks assessing cortical dysfunction.
Whether part or the full range of cognitive deficits in PD should be called MCI, is a matter of debate. The very concept of MCI - that is the emergence of cognitive deficits heralding dementia-- is a concept exported from AD and might demonstrate less operative in PD. The problem of preclinical detection of dementia is substantially different in PD and AD. The key is to identify healthy elderly subjects with subtle cognitive changes well before overt signs of dementia occur. The problem in PD is to select, among a broad range of neuropsychological abnormalities those predictive of significant functional impairment and later dementia. Central to this problem is the course of cognitive decline in PD. The emergence of dementia is common as PD progresses. One systematic review reported a 25–30% point prevalence of dementia in patients with PD. Longitudinal studies have established that the risk of dementia is four to six times greater in PD patients than in the general population, and one community-based study reported a cumulative prevalence of PDD of 78% at eight years’ follow-up. It is incompletely known whether with the progression of PD deficits in attention and executive function can be sufficiently severe to qualify as dementia or whether cortical deficits are mandatory. The presence and progression of cognitive impairments were mainly assessed using a range of neuropsychological batteries that have wide variation, low sensitivity and poor specificity for PD. Methodological differences in tools used to assess cognitive deficits precluded direct comparisons between studies and, consequently, have made it difficult to operationally define ‘mild cognitive impairment’ in PD. To avoid further confusion, the PD community should accord whether the term MCI is employed for the whole spectrum of cognitive deficits seen along the course of PD or it is reserved for the ‘malignant’ type of CI perceived by the patient or family that poses a PD patient in an imminent risk of further cognitive decline enough to qualify as PD dementia. In that sense, it is difficult to establish an operational definition of what can be called ‘mild cognitive impairment in PD’: only executive deficits? Executive plus memory deficits? Executive plus visuospatial alterations? Executive plus language deficits? A combination of all? The compromise solution of using zeta scores, for instance using 1.5 Standard Deviations with smaller series may categorize as cognitively unimpaired many PD patients with otherwise relatively subtle cognitive defects. Which should be the name for the cognitive defects of these patients?
The early recognition and accurate diagnosis of cognitive impairment specifically leading to dementia in PD, is a challenge faced by physicians and caregivers today, along with the need for earlier intervention and disease management. Specific PD-testing batteries would be useful for discriminating between different types of cognitive dysfunction, measuring and monitoring neuropsychological variables highly predictive of subsequent development of dementia, comparing results generated by different research groups, and assessing clinical responses of PD patients during therapeutic trials. Recent data adds further support to the notion that the transition from mild cognitive impairment to dementia in PD patients is characterised by the addition of cortical-type cognitive deficits atop a prominent and progressive frontal- subcortical dysfunction.
ALEMTUZUMAB
J. Losy
Department of Clinical Neuroimmunology, Chair of Neurology, University School of Medicine, Neuroimmunological Unit Institute of Experimental and Clinical Medicine Polish Academy of Sciences, Poznan, Poland
Alemtuzumab is a humanized monoclonal antibody directed against CD52, a cell surface antigen expressed mainly on T and B lymphocytes, monocytes and macrophages. Alemtuzumab depletes CD52 cells through complement-mediated lysis, antibody-dependent cell toxicity and induction of apoptosis. Treatment with alemtuzumab results in profound lymphopenia. This monoclonal antibody was used to treat B- cell lymphomas and chronic lymphocytic leukemia. Alemtuzumab has been also found to be effective in the treatment of multiple sclerosis. Two open labeled studies have been performed by the group from Cambridge in relapsing-remitting and secondary progressive MS. In 22 patients with RR MS (17 drug naive patients and five who had already failed treatment with IFN-beta) a profound reduction of relapse rate by 91% has been shown (from 2.2 annual relapse rate before treatment to 0.19 during follow up for a mean of 29 months. The same group of patients in a year before treatment showed increase of disability by +2.2 EDSS points. After treatment which is an extremely interesting finding, sixteen out of twenty two patients at one year had an improved EDSS. All but one of the others was stable. The mean effect was an improvement by -1.4 points compared to baseline. This improvement has been observed also after 24 months. In 36 patients with secondary progressive MS the treatment ,which consisted of 5 doses of 20 mg alemtuzumab, produced in the year following treatment a sharp decline in the relapse rate and marked reduction in the number of contrast-enhanced lesions. Most of patients had stable EDSS. In following years a progression of disability was observed. After a mean 7.5 yeas of follow-up only 4/36 patients remained stable and remaining 32 subjects continued disability progression. As both RR and SP studies were open label and did not use a control group, must be interpreted with caution. Alemtuzumab was generally well tolerated. Adverse reactions included infections ( cases of measles, herpes zoster, varicella zoster) , autoimmune hyperthyroidism (Graves disease) reported in 27% of patients , acute renal failure as a result of Goodpasture' s syndrome. High number of Graves disease seems to be associated with quicker recovery of CD8 lymphocytes, which are implicated in the pathogenesis of autoimmune thyroiditis. Another important study has been recently published comparing alemtuzumab treatment with interferon beta 1a. In this phase 2 randomized, blinded trial 334 patients with relapsing- remitting MS received subcutaneous interferon beta 1a three times weekly (111 patients) and 223 were assigned to receive alemtuzumab at a dose of 12 mg per day or 24 mg per day. Alemtuzumab was given by intravenous infusion on 5 consecutive days during the first month and on 3 consecutive days at months 12 and 24. Alemtuzumab significantly reduced the rate of sustained accumulation of disability as compared with interferon beta 1a (9% vs 26.2%) and reduced the annualized rate of relapse (0.1 vs 0.36). The mean disability score improved by 0.39 points in the alemtuzumab group, confirming previous findings from open label study, and worsened by 0.38 point in the interferon beta 1a group. In the alemtuzumab treated patients the lesion burden seen on T2 MRI was reduced in comparison with interferon beta 1a group. From month 12 to month 36, brain volume increased in the alemtuzumab group and at the same time decreased in the interferon beta 1a group. A possible mechanism may be the secretion of neurotrophins by lymphocytes regenerated after administration of alemtuzumab. Adverse events in the alemtuzumab group additionally to infections and thyroid disorders included several cases of thrombocytopenic purpura. The results of this study show not only some advantages of alemtuzumab therapy in comparison with interferon beta 1a treatment but also indicate that impeding inflammation early in the disease reduces the extent of neurodegeneration that is associated with disease progression , giving an important information for answer to one of most fundamental questions about relation between inflammation and neurodegeneration in MS pathology.
PROTEOMICS AS A TOOL IN BIOMARKER RESEARCH FOR PARKINSON'S DISEASE
K. Marcus
Department Functional Proteomics, Medizinisches Proteom-Center, Ruhr-University Bochum, Germany
Proteins and peptides present within clinical samples represent a valuable library of information regarding the ongoing processes within cells and tissues in health and disease. Many of the proteins associated with natural biofluids such as blood, serum, plasma or CSF can be linked with biological processes that are of clinical importance. This linkage typically falls into two different categories: either a direct association of functional expression of a protein to normal biological pathways or an indirect association relating the presence of the protein in the sample with a given biological process, i.e. biomarker. The research field of biomarker discovery linked to specific diseases and therapeutic treatment is an important emerging field providing a real benefit in clinical research. For greatest clinical use, a biomarker should be measurable in an easily accessible body fluid such as serum, plasma, or urine.
In regard to PD, many proteins are known that could be the cause or the consequence of the pathomechanism. In the last years it became obvious that the expressed range of proteins or above all their potential modifications cannot be predicted from the genetic information alone. Several biomarkers for PD were already identified by genetic approaches. Indeed, in the last years it turned out that the majority of neurodegenerative disorders including PD seem to be caused and/or modified by multiple genes rather than by a malfunction of a single protein. These coherences could only be detected using proteomic approaches. Additionally, in contrast to e.g. RNA the proteins of given cells or tissue can be fractionated allowing a very area specific analysis and consequently to gain even more information in a quantitative as well as qualitative sense. In consequence, proteomics emerged as a key technology to identify novel proteins, to characterize their isoforms based on numerous post-translational modifications, and to unravel protein regulation, e.g., in healthy and diseased states. When comparing the proteome of two states e.g. healthy vs. diseased tissue, distinct protein alterations correlating with the disease state are identified.
Brain affecting diseases such as PD most commonly affect elderly people and give rise to severe and slowly progressing neurodegeneration. Since there is neither an effective pharmaceutical treatment available to cure PD, nor to reliably diagnose it at a pre-symptomatic stage, proteomics may contribute to the identification of new diagnostic protein biomarkers and novel therapeutic targets in the future.
In my talk I will focus on the application of proteomics on the investigation of biomarkers for PD addressing the question on which proteomic strategies might be best to follow underlining advantages as well as limitations of some of the existing strategies.
PATIENTS WITH MEDICATION OVERUSE HEADACHE (MOH) SHOULD UNDERGO WITHDRAWAL PRIOR TO INITIATING PREVENTIVE MEDICATION
N. Mathew
Houston, Texas, USA
Combination analgesics containing caffeine, Butalbital containing compounds, opioids, ergotamine, and triptans can transform episodic migraine into chronic daily headache (CDH). Prospective epidemiological studies reported that frequent use of analgesics (once a week or more) as a risk factor for chronic headache, relative risk (RR) for chronic migraine being 13.3 and chronic non migrainous headache being 6.2. Daily use of opioids for nonheadache conditions (e.g.: to control bowel motility in colectomy patients) increases the risk of migraine transforming to CDH.
EVIDENCE FOR THE NEED OF WITHDRAWAL: In 1990, before the triptan ERA, we reported the treatment outcomes in 200 patients with MOH.5 116 (58%) of them were on prophylactic medications. The effects of continuing symptomatic medications, their withdrawal, and adding or changing prophylactic medications after withdrawal were studied. Mere discontinuation of medication overuse itself resulted in significant improvement. Continuous use of symptomatic medications, unchecked, resulted in very insignificant improvement in spite of dietary and behavioral treatment. Withdrawal enhanced the efficacy of prophylactic agents.
There were no triptan overusers in this group, majority overused combination analgesic containing caffeine, codeine and Butalbital.
Bigal et al in 2004 reported that patients who were detoxified and concurrently put on prophylaxis had substantially more reduction in headache days (73.7% vs. 17.2%) and headache scores (frequency X severity) compared to those not detoxified.
Zeeberg, Olesen and Jensen in 2006 reported the benefits of withdrawal from overused medications as 1) reduction in the frequency and severity of headaches, 2) recovery of therapeutic responsiveness. Former nonresponders to preventive medications had a 49% decrease in headache frequency when they were put on prophylaxis after withdrawal.
NOT ALL MOH ARE THE SAME: MOH is a complex phenomenon, the mechanism of which is poorly understood. There are differences in the occurrence of MOH depending on:
1. Type of medications overused
2. Frequency of use
3. Comorbid conditions
In the United States, combination analgesic containing caffeine and Butalbital and opioids are overused more frequently than triptans. The reason for this is partly because the number of triptans dispensed is limited and they are much more expensive.
There is a distinct difference between triptan overuse and overuse of other analgesics including opioids.
1) Triptan overusers have less severe and shorter duration medication withdrawal headache.
2) Less prone to relapse (21% vs. 71%).
3) Recent epidemiological studies clearly showed, while migraine progression from episodic to chronic migraine can occur on triptans in those with high frequency migraines 10-14 days/month, for those with low frequency migraine triptan use did not seem to cause migraine progression.
4) Triptan MOH differs from other MOH by the fact there is more increase in the number of migraines, rather than occurrence of more nondescript or nonmigrainous headache.
It is therefore obvious that triptan MOH should be managed differently than MOH due to other agents; MOH from opioids and combination analgesic containing Butalbital and caffeine. Withdrawal headaches in this group are more severe and prolonged and associated with other withdrawal symptoms such as restlessness, sleeplessness, diarrhea, etc.
OPIOID INDUCED HYPER ANALGESIA (OIH): There is increasing clinical and experienced evidence to support the clinical entity of opioid induced hyper analgesia in those who use opioids frequently. Clinical features include worsening of the frequency and severity of headache, allodynia, diffuse spreading of what was initially experienced as more focal headache and the need for increasing the dose of opioids.
For migraine patients, exposure to opioids may impede the efficacy of non opioid analgesics. In a study by Jakubowski et al, 64 – 71% of migraine patients achieved freedom from acute migraine pain and allodynia within one hour of ketorelac infusion. Those who failed to respond had a history of frequent opioid intake.
FLIMSY EVIDENCE FOR NOT RECOMMENDING WITHDRAWAL: My opponent in this debate, Professor Schonen’s whole argument that acute medication withdrawal is not necessary, is based on a single under powered subset analysis of a small study published by Diener et al (2007).
You can judge yourself the validity of the conclusion of that study on which Professor Schonen will base his recommendations, refuting a quarter century old solid careful clinical observations and conclusion shared by clinicians.
The facts of the study are as follows:
1) A mere 23 patients with MOH received prophylactic therapy.
2) Authors themselves admit that it was under powered.
3) 61% of the patients were triptan overusers and not the hard core complex MOH with opioids and combination analgesics containing, Butalbital, caffeine and opioids. As I discussed, triptan MOH should be considered separately and differently. They are much easier to treat.
4) Preventive therapy did not significantly reduce medication overuse compared to placebo.
5) There was no change in MSQ and HIT6.
6) This study did not have a control group who underwent withdrawal before preventive meds were started. That control withdrawal group would have shown robust results. Without that information, the argument has no validity.
We recently did a post-HOC analysis of MOH patients in the chronic migraine preventive study using Topiramate conducted in the US. MOH with triptans, simple analgesics and combination analgesic containing opioids, Butalbital and caffeine were studied. The differences in mean change from baseline in migraine/migrainous days between Topiramate treated group and placebo treated group of MOH, were statistically significant only for subjects overusing triptans.
No experienced clinician would advocate withdrawal as the sole solution of MOH. Withdrawal should be simultaneously accompanied by initiation of prophylactic treatment, bridge therapy to counter the transient increases in headache and other withdrawal symptoms during withdrawal, and supportive behavioral therapy. But to assume that withdrawal is not necessary based on poor evidence, is rather irresponsible. Patients who are withdrawn respond to preventive medications a lot better.
References: Capobianco DJ, Swanson JW, Dodick DW. Medication-induced (analgesic rebound) headache: Historical aspects and initial descriptions of the North American experience. Headache. 2001; 41:500-502.May; Dodick D, Freitag F. Evidence-based understanding of medication-overuse headache: Clinical implications. Headache. 2006; 46 (Suppl. 4):S202-S211; Zwart JA, Dyb G, Hagen K, Svebak S, Holmen J. Analgesic use: A predictor of chronic pain and medication overuse headache: The Head-HUNT Study. Neurology. 2003; 61:160-164; Wilkinson SM, Becker WJ, Heine JA. Opiate use to control bowel motility may induce chronic daily headache in patients with migraine. Headache. 2001; 41:303-309; Mathew NT, Kurman R, Perez F. Drug induced refractory headache – clinical features and management. Headache. 1990; 30:634-638; Bigal ME, Rapoport AM, Sheftell FD, Tepper SJ, Lipton RB. Transformed migraine and medication overuse in a tertiary headache centre – clinical characteristics and treatment outcomes. Cephalalgia. 2004; 24:483-90; Zeeberg P, Olesen J. Jensen R. Discontinuation of medication overuse in headache patients: recovery of therapeutic responsiveness. Cephalalgia. 2006;26:1192-8; Katsarava Z, Fritsche G, Muessig M, Diener HC, Limmroth V. Clinical features of withdrawal headache following overuse of triptans and other headache drugs. Neurology. 2001;57:1694-1698; Katsarava Z, Muessig M, Dzagnidze A, Fritsche G, Diener HC, Limmroth V. Medication overuse headache: Rates and predictors for relapse in a 4-year prospective study. Cephalalgia. 2005; 25:12-15; Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology 2008, 71:1821-1828; Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006; 104:570-587; Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep. 2006;10:67-70; Watkins LR, Hutchinson R, Johnston IN, Mauer SF. Glial novel counter-regulators of opioid analgesia. Trends Neurosci. 2005; 12:661-669; Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450- 455; Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850-861; Diener HC, Bussone G, Van Oene J, Lahaye M, Schwalen S, Goadsby P; TOPMAT-MIG-201(TOP-CHROME) Study Group. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27:814-823; Dodick DW, Silberstein S, Mathew N, Hulihan JF, Ascher S, Xi N, Morein JD, Wright P, Biondi DM, Greenberg SJ. Evaluating the effect of Topiramate treatment in subjects with chronic migraine and medication overuse. In Press
THE USE OF VITAMINS IN MIGRAINE: CON
A. Mauskop
USA
Out of respect to my colleague and her work I will not directly argue against the use of riboflavin, cyanocobalamine, vitamin D, or any other vitamins, which incidentally I recommend to some of my migraine patients.
Instead, to counter her point, I would like to present arguments for the use of magnesium and CoQ10 in the treatment of migraine headaches.
According to Wikipedia, coenzyme Q10 (also known as ubiquinone, ubidecarenone, coenzyme Q, and abbreviated at times to CoQ10, CoQ, Q10, or simply Q) is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the isoprenyl chemical subunits.
This oil-soluble vitamin-like substance is present in most eukaryotic cells, primarily in the mitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, generating energy in the form of ATP. Ninety-five percent of the human body’s energy is generated this way.
P. S. Sándor, L. Di Clemente, and G. Coppola in their paper Efficacy of coenzyme Q10 in migraine prophylaxis: A randomized controlled trial (Neurology 2005;64:713
-715) presented results of a double-blind, randomized, placebo- controlled trial which involved 42 patients. Half of the patients received CoQ10, 100 mg TID and the other half, placebo. They found that 50% responder rate for attack frequency was 14.4% for placebo and 47.6% for CoQ10.
Hershey AD and his colleagues (Headache 2007;47:73-80) published a paper, Coenzyme Q10 deficiency and response to supplementation in pediatric and adolescent migraine. (Headache 2007;47:73-80). They measured CoQ10 levels in 1550 patients and found that 32.9% were deficient. Supplementation with 1-3 mg/kg/day improved CoQ10 levels (p<.0001), headache frequency improved from 19.2 to 12.5, p<.001 and headache disability improved from 47.4 to 22.8, p<.001.
Clearly, CoQ10 is a much stronger contender in battle to relieve migraine headaches than riboflavin, vitamin D, cyanocobalamine, or any other vitamin. However, I reserve my strongest arguments for the use of magnesium for the prophylaxis of migraine over any vitamin.
Magnesium is an essential cation that plays a vital role in multiple physiological processes, regulating tissue and cell functions. Deficits in magnesium can be seen in any chronic medical illness, including cardiovascular disease, diabetes, pre-eclampsia, eclampsia, sickle cell disease and chronic alcoholism [1]. Symptoms of magnesium deficiency include premenstrual syndrome, leg muscle cramps, coldness of extremities, weakness, anorexia, nausea, digestive disorders, lack of coordination, and confusion. Magnesium may be involved in migraine pathogenesis by counteracting vasospasm, inhibiting platelet aggregation, and the stabilization of cell membranes [2]. Its concentration influences serotonin receptors, nitric oxide synthesis and release, inflammatory mediators, and various other migraine-related receptors and neurotransmitters [3]. Magnesium also plays a role in the control of vascular tone and reactivity to endogenous hormones and neurotransmitters, via its relationship with the NMDA receptor [4,5]. In the brain, a deficiency of magnesium results in contraction and potentiation of vasoconstrictors. [6].
Studies have shown that migraineurs have low brain magnesium during migraine attacks [7] and may also have a systemic magnesium deficiency [8,9]. Furthermore, a deficiency of magnesium may play a particularly important role in menstrual migraine [10]. There have been 2 double-blind, placebo-controlled trials which have shown that oral magnesium supplementation is effective in headache prevention [11,12]. A third study [13] was negative, but this result has been attributed to the use of a poorly absorbed magnesium salt, since diarrhea occurred in almost half of patients in the treatment group. Intravenous magnesium has been shown to be an effective migraine abortive agent in patients with low ionized magnesium levels, but not in those with normal levels [14]. The most commonly reported adverse effect of magnesium supplementation is diarrhea.
References: 1 Laires MJ, Monteiro CP, Bicho M. Role of cellular magnesium in health and human disease. Front Biosci 2004; 1:262-76. 2. McCarty MF. Magnesium taurate and fish oil for prevention of migraine. Med Hypotheses 1996; 47: 461-6. 3. Bianchi A, Salomone S, Caraci F, et al. Role of magnesium, coenzyme Q10, riboflavin, and vitamin B12 in migraine prophylaxis. Vitamins and Hormones 2004; 69: 297-312. 4. Turlapaty PDMV, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science 1980;208:198-200.
5. Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A. Mg2+- Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol 1987;65:729-745. 6. Altura BT, Altura BM. Withdrawal of magnesium causes vasospasm while elevated magnesium produces relaxation of tone in cerebral arteries. Neuroscience Lett 1980;20:323- 327. 7. Ramadan NM, Halvorson H, Vande-Linde A. Low brain magnesium in migraine. Headache 1989; 29: 590-3. 8. Trauinger A, Pfund Z, Koszegi T, Czopf J. Oral magnesium load test in patients with migraine. Headache 2002; 42: 114-9. 9. Mauskop A, Altura BM. Role of magnesium in the pathogenesis and treatment of migraine. Clin Neurosci 998; 5: 24-7. 10. Mauskop A, Altura BT, Altura BM. Serum ionized magnesium in serum ionized calcium/ionized magnesium ratios in women with menstrual migraine. Headache 2001; 42: 242-8. 11. Facchinetti F, Sances G, Borella P, Genazzani AR, Nappi G. Magnesium prophylaxis of menstrual migraine: effects on intracellular magnesium. Headache 1991; 31: 298-301. 12. Peikert A, Wilimzig C, Kohne-Volland R. Prophylaxis of migraine with oral magnesium: results from a prospective, multicenter, placebo-controlled and double-blind randomized study. Cephalalgia 1996; 16: 257-63. 13. Pfaffenrath V, Wessely P, Meyer C, et al. Magnesium in the prophylaxis of migraine- a double-blind placebo-controlled study. Cephalalgia 1996; 16: 436-40. 14. Mauskop A, Altura BT, Cracco RQ, Altura BM. Intravenous magnesium sulfate relieves migraine attacks in patients with low serum ionized magnesium levels; a pilot study. Clinical Science 1995; 89: 633-6.
DEEP BRAIN STIMULATION FOR NEURORECOVERY
M. Mehdorn
Department of Neurosurgery, University Clinics of Kiel, Germany
Recent advances in Deep Brain Stimulation (DBS) with experiences in a multitude of specific targets have brought a variety of improvements to patients with movement disorders, possibly also cluster headache, seizures, severe psychiatric disorders and others. Also neuro- augmentative possibilities came into the reach of surgery.
While the exact mechanisms of action of DBS are still not quite understood, it has repeatedly been questioned whether the positive effects of DBS are due only to acute alterations of the electrical fields around the electrodes or whether additional long-term effects might be derived from a chronic application of this technique. This question also relates to the long-term efficacy of the method of DBS, to a restorative – regenerative effect of DBS on one hand and to the possibility of unwanted side effects on the other hand.
Time course of DBS effects depends, to a great extent, on the disorder treated: While some symptoms of Parkinson’s disease can be rapidly changed when a well placed electrode is turned on and off, the beneficial effects of DBS may take quite some time before they become clinically relevant in dystonia patients, and this delay is even more pronounced in patients with cephalgia. When clinical conditions such as vegetative status are taken care of this time until symptoms may improve can last several months.
Modes of actions and the potentials of DBS incl. unwanted side effects are delineated based on own experiences as well as on a review of the pertinent literature. Modes of action may include immediate changes of electrical fields e.g. in the basal ganglia as well as chemical alterations and changes of early immediate genes mediating long-term alterations in loops affected by DBS. These mechanisms may be responsible for the slow part of the improvement curves.
The future role of multi-modality image-guided neurosurgical procedures will be discussed based on some examples – much cooperative research is needed to fully explore their potentials for the future, but also ethical aspects will need to be taken care of. Most probably a gradual switch from purely medico-conservative treatment of some disorders to inclusion of well analyzed and study-evaluated neurorestorative procedures into the treatment armamentarium will be seen in the coming years.
BIOMARKERS IN PERSONALIZED AD CARE: OPPORTUNITIES, CHALLENGES, APPROACHES
M. Memo
Department of Biomedical Sciences and Biotechnologies, University of Brescia Medical School, Brescia, Italy
In 2001, a National Institutes of Health working group has defined biological markers or biomarkers as “biological substances or images that are objectively measured and evaluated as an indicator of normal biological processes, pathological processes, or pharmacological responses to therapeutic intervention”. In this contest, biomarkers can provide indications of both the potential effectiveness and the potential hazards associated with drug treatment. They can also be used to make decision about whether to develop a drug as well as to screen compounds for toxicity before enter clinical trials. In addition, biomarkers may give substantial information on onset and progression of specific pathologies allowing accurate diagnosis and therapeutic intervention.
Although biomarkers can provide predictive information based solely on its intensity and organ response, they have their greatest value when they unveil pathophysiological mechanisms. Yet, regardless of whether such mechanistic insights are gained, reliable information that can distinguish who is at risk and who will benefit is valuable. This capability forms the basis for the concept of personalized medicine, which employs biomarkers to stratify populations into smaller groups according to such differences in benefit and risk.
Although quite relevant and scientifically recognized in several fields, including cardiovascular, tumour, and haematological diseases, biomarkers are now facing to neurodegenerative diseases such as Alzheimer’s disease (AD). The development of biomarkers for AD has gained great and broad interest among scientists, economists and biotechnological companies. In fact, the predictive value of biomarkers is characterized by the criteria used for assessing them, for example, sensitivity, specificity, reliability, and discrimination. But this is not enough. Equal relevant are the cost, invasiveness, time and technology required for evaluation, as well as the compliance by both healthy and affected population. Thus, the scientific validation solely does not imply a large diffusion of biomarkers to the community and does not guarantee a social benefit. The development of needed information about biomarkers has to be beyond the scope of individual company or academic institution. Furthermore, national or international Health Agencies are neither equipped nor funded to conduct such research. Accordingly, incentives are needed to encourage research groups to overcome traditional barriers of secrecy and intellectual property. Incentives could be helpful in translating the results of basic research into biomarker applications that have an impact on health care.
In the AD field there are two additional concepts that deserve to be mentioned: the concept of “peripheral” biomarkers and the concept of “early diagnosis”. In fact, early diagnosis is an important first step in management of AD. The prediction of the incipient disease allows an earlier pharmacological approach. In this contest, identification of new putative biomarkers can increase the accuracy of clinical diagnosis but, more importantly, make earlier diagnosis possible. In the last 20 years independent scientific groups have looked for biological and biochemical alterations in peripheral tissues of AD patients, because of their easy accessibility. The concept of peripheral biomarker implies the involvement of many cell phenotypes in the disease and suggests that AD can be considered as a systemic disease. The major difficulty in the universal acceptance of a peripheral biomarker for CNS disease is its relevance in the pathogenesis of the illness. The study of accredited biomarkers found in CSF, has failed when the same were looked for in periphery, suggesting that, if metabolic and biochemical alterations occur in peripheral cells, they do not necessarily mirror event occurring in the AD brain.
CHARACTERISATION OF THE AUTOIMMUNE ANTIBODY REPERTOIRE OF PARKINSON’S DISEASE PATIENTS BY SYSTEMATIC SCREENING OF PROTEIN ARRAYS
H. Meyer, A. Kowald, A. Lueking, D. Woitalla, L. Tenenberg Grinberg, M. Gerlach, P. Riederer, H., E. Nordhoff
Medizinisches Proteom- Center, Ruhr-University Bochum, Germany; Protagen AG, Dortmund, Germany; Neurologische Universitätsklinik der Ruhr-Universität Bochum, St. Josef Hospital, Germany; Clinical Neurochemistry (National Parkinson Foundation Center of Excellence Research Laboratory), Department of Psychiatry and Psychotherapy, University of Würzburg, Germany
Dept. of Pathology – University of São Paulo Medical School, Brazil
The diagnosis of Parkinson disease (PD), a degenerative disorder of the central nervous system, relies on the recognition of clinical symptoms appearing in a late stage of pathogenesis. At this stage of PD only the treatment of the clinical symptoms is feasible, a therapy that is capable to stop the progression of the disease is still not available. Therefore, the identification of molecular markers allowing an early diagnosis of PD is urgently needed.
Following the hypothesis that the progression in Parkinson’s disease may be caused by a chronic autoimmune response, we have characterized the autoimmune antibody repertoire of PD patients by serum hybridization of protein arrays in order to determine the potential of selected autoimmune antibodies to act as molecular markers for PD.
We utilize the UNIarray-technology that combines the advantages of protein arrays with the large-scale expression of proteins from existing cDNA libraries to screen >120 serum samples of PD patients against 10 000 heterologously expressed human proteins arranged on a macroarray. This approach resulted in the identification of ~150 autoantigens that represent putative disease markers. First results indicate that such a diagnostic protein array allows differentiating PD patients from non- affected ones.
To validate these molecular markers an iterative screening procedure was developed allowing a stepwise reduction of candidate proteins with concomitant increase of quality and purity. We are currently analysing a much larger cohort of PD patients and non-PD patients in order to define a smaller subset of marker proteins that allows the set up of a blood-based in vitro diagnostic assay.
NEWLY EMERGING THERAPIES: DO THEIR POTENTIAL BENEFITS OUTWEIGH THE RISKS? YES
R. Milo
Department of Neurology, Barzilai Medical Center, Ashkelon, Faculty of Health Sciences, Ben- Gurion University of the Negev, Israel
Multiple sclerosis (MS) affects 2.5 million people around the world. It is the leading neurological cause of disability in young and middle aged adults. Disease severity is highly variable – from asymptomatic or very mild (truly benign) forms compatible with normal life, to rapidly progressive cases or acute fulminant MS of the Marburg type causing death within a short time. Continuous disease activity leads to early axonal damage, brain atrophy and cognitive dysfunction that are evident from disease onset. Typically, the initial, predominantly inflammatory stage of the disease is followed by a later stage characterized by diffuse neurodegeneration that is expressed by progressive and irreversible disability. Certain populations such as blacks or Asians with optico-spinal MS may have more severe disease with earlier age of onset, more rapid progression, and more advanced physical and cognitive impairment. MS strikes young people and may interfere with academic achievements, employment, career or family life. Common chronic and disabling symptoms such as fatigue, gait difficulties, ataxia, tremor, spasticity, urinary incontinence, depression and cognitive impairment have a profound impact on social roles, productivity, quality of life and well- being of patients and their families: MS patients frequently collect disability benefits and social welfare and use more health-care resources than the general population; Their ability to continue in gainful employment or to maintain social contacts and leisure activities is limited and correlates with the course and severity of the disease and cognitive function; Their quality of life is significantly affected; They are 5-10 times more likely to attempt or commit suicide than the general population (more than patients with other common neurological disorders); Most careers of MS patients report symptoms that clearly relate to organic pathologies, anxiety and symptoms of depression, and their professional careers are also adversely affected by the patient’s illness. The economic cost of MS to society is also great. According to e recent Australian economic analysis, the burden of disease — the suffering and premature death experienced by people with MS — is estimated to cost an additional 8968 DALYs (years of healthy life lost), with two thirds attributable to disability and one third to premature death.
The need to treat and control MS activity is obvious. In the absence of a cure for MS, treatment is expected to prevent relapses, suppress disease activity, slow down disease progression and accumulation of disability, delay the progressive phase and improve quality of life. Available first line immunomodulators for relapsing MS (interferons and glatiramer acetate, GA) are only partially effective. Most patients experience disease activity and progression on these treatments: 62-75% relapses within 2 years and 20-27% worsen by ≥1 point on EDSS within 2 years. Moreover, some MS patients cannot tolerate or decline injectable disease modifying therapies, and patients with highly active disease represent a specific problem in MS management. Recent advances in our understanding of the immune pathogenesis of MS have resulted in the emergence of numerous new treatments that may better address these needs. In addition to the approved natalizumab, these include other monoclonal antibodies (alemtuzumab, daclizumab, rituximab), oral agents (cladribine, fingolimod, BG12, teriflunomide, laquinimod) and others. Most of these agents showed higher magnitude of clinical and MRI efficacy than interferons or GA in phase II-III placebo-controlled trials. Other comparative or combination trials confirmed the superiority of fingolimod over avonex, of alemtuzumab over rebif, or of natalizumab combined with avonex over avonex alone. The profound effect of some of these agents on disease activity introduced the new ambitious “disease free” concept (no relapses, disability progression, Gd. enhancement or new T2 lesions on MRI) in a significant proportion of patients, and the benefit of natalizumab in highly active patients or in those who failed previous therapies is even higher. These agents have some other advantages such as oral administration or infrequent dosing which may enhance compliance, improved quality of life and a favorable cost- effectiveness. On the other hand, new and serious adverse events have also emerged with these powerful therapies: Opportunistic and other infections (especially PML), malignancies, secondary autoimmunity and others.
Calculating the number needed to treat (NNT) and number needed to harm (NNH) for various treatment-related variables may help in evaluating the benefit-risk ratio for these agents: lower NNT indicates a better response to treatment, higher NNH indicates a better safety profile, and a better benefit-to-risk ratio may be expressed as higher NNH/lower NNT.
It is clear that most new therapies provide better benefit than the older ones and are better tolerated (higher NNH for withdrawal from the study because of adverse events). Most of their serious adverse events that may raise safety concerns are rare and have high NNH’s, indicating low risks. Even with the 1:1000 rate for PML that was guesstimated when the drug was reintroduced to the market (which is much higher than the current risk), a more than sevenfold increase in actual risk of PML is required to decrease natalizumab’s health gain below that of interferon - 1a, and there are additional gains in health associated with natalizumab therapy beyond those already achieved with current therapies, according to a recent quantitative risk-benefit analysis. Moreover, most MS patients indicate they are willing to accept risks in exchange for clinical efficacy. Taken together, the benefit-to risk ratio for these newly emerging therapies, at least for the short term, is more than acceptable. This makes them potential first or second line therapies for MS.
Recognizing the potential risks associated with these new therapies and which patients may benefit or be at greater risk from each drug should lead to the appropriate clinical vigilance. For the benefit of our MS patients, we should take the extra efforts and the appropriate measures of clinical vigilance in order to further minimize risk and maximize benefit.
TRIPTANS MUST BE GIVEN EARLY WHEN THE HEADACHE IS MILD FOR THEM TO WORK WELL
D. Mitsikostas
Athens Naval Hospital, Athens, Greece
Even before the introduction of triptans in early 1990s, migraineurs have been reporting that headache is more sensitive to any symptomatic treatment when it is mild, in the beginning of the attack. And they consistently report this not only for triptans, but also for simple analgesics, NSAIDs, ergot alkaloids, or opioids, alone or in combinations. Whether this is a fact or not has been tested several times, by different investigators, following different methodologies, resulting always into the same conclusion. Early migraine treatment is beneficial. It is believed that this is associated to central sensitization within the brainstem neurons. When the compound is offered early into the brainstem, it prevents sensitization of cells and limits the propagation and progression of pain signaling (Burstein et al., Brain 2000; 123: 1703-9). At least four randomized clinical trials addressed the question of early intervention in migraine management and analysis of data from all studies showed that naratriptan, rizatriptan, frovatriptan and almotriptan are more effective when given early than later within a migraine attack. These are the data:
(1) Naratriptan: when given during 63 cases of prodrome migraine phase in 20 migraineurs at a dose of 2.5 mg prevented headache at 38 cases (60%) compared to 59/59 cases of headache occurrence (100%) at baseline. In cases that despite treatment headache occurred, pain was mild (Luciani et al., Cephalalgia 2000; 20:122-16).
(2) Rizatriptan: A total of 1919 migraineurs with 3450 migraines were evaluated, of which 1369 episodes were early treated with rizatriptan 10mg and 2081 delayed treated. Compared to delayed-treated episodes, episodes wherein patients took rizatriptan early were 1.33 times more likely to be characterized by onset of headache relief within 30 minutes post-treatment (95% confidence interval [CI], 1.11 to 1.60), 1.32 times more likely to result in a largely symptom-free state (95% CI, 1.12 to 1.57), and 1.34 times more likely to be associated with a return to normal activities (95% CI, 1.13 to 1.58) within 1 hour post-treatment. No significant difference between early-treated and delayed-treated episodes was observed with respect to the likelihood of there being no or mild headache 2 hours post-treatment (odds ratio, 1.05; 95% CI, 0.90 to 1.24; Hu et al., Headache 2002;42:16-20).
(3) Frovatriptan: in 241 migraineurs who each treated at least two migraine attacks, frovatritpan given at the onset of mild headache was superior than frovatriptan given when headache was moderate/severe two hours later (57% vs. 46%, Cady et al., Curr Med Res Opin 2004;20:1465-72).
(4) Almotriptan: 403 migraineurs were treated with almotriptan 12.5mg in a randomized; four arm RCT to compare outcomes after headache onset (mild/early) with outcomes when pain had become moderate or severe. 53% of the mild/early group and 37.5% of the moderate/severe group were pain free at 2 hours post treatment (p=0.02), whereas the corresponding proportions in the placebo groups were 24.7& and 17.5% (p<0.01, Goadsby, Cephalalgia 2008; 28; Suppl 2:36-41).
Post hoc analyses with other triptans also found similar results. To assess the dose-efficacy relationship of oral sumatriptan 50mg and 100mg given early for mild pain, data from six RCTs were pooled for analysis. In all efficacy parameters including pain free and sustained pain free the drug was superior to placebo (Winner et al., Clin Ther 2005; 27:1785-94). There is only one RCT with contradictory results. Sumatriptan when given early and subcutaneously was not superior compared to late subcutaneous administration in 20 adults migraineurs, in terms of pain free, pain severity at one and two hours post-treatment, area under the curves from injection to pain free or in headache recurrence after injection. Participants claimed that their medication was as effective when given early as when given late in the course of the attack. The investigators speculated that the discrepancy between their findings and the findings from other studies with oral triptans is related with pharmacokinetics (Linde et al., Cephalalgia 2006; 26:113-21). Apparently, the results of this study confirm the clinical experience of subcutaneous sumatriptan as a rescue treatment in migraine. Although skepticism and criticisms have been reported in the literature that only patients with particularly severe migraines and in whom attacks are always characterized by rapid progression of pain and other symptoms should be advised to take a triptan as early as possible (Moschiano et al., Neurol Sci 2005; 26 Suppl 2:s108-10) the above mentioned data from the four RCTs with oral triptans are unquestionable and highly considerable into the clinical practice. Potential risks, such as medication overuse should take into account however.
SONOTHROMBOLYSIS IN ACUTE ISCHEMIC STROKE
C. Molina
Spain
Experimental and clinical studies have consistently demonstrated the capability of ultrasound (US) to enhance enzymatic thrombolysis. US application increases the transport of tPA into the thrombus, promotes the opening and cleaving of the fibrin polymers, and improves the binding affinity of tPA to fibrin. In an observational pilot trial of combined therapy with 2-MHz continuous US monitoring and intravenous tPA in 55 patients with a documented MCA occlusion treated <3 hours of stroke onset, complete recanalization at 2 hours of tPA bolus was achieved in 36% of patients. In a small study using transcranial color- coded sonography (TCCS), 32 patients were randomly allocated to be treated with combined TCCS and intravenous tPA or tPA alone <6 hours of symptom onset. Combined treatment was associated with higher rates of recanalization but also with a higher rate of ICH. CLOTBUST, a phase 2 multicenter randomized trials, recently demonstrated that 2-hour continuous monitoring with 2-MHz TCD, a commercially available device widely used for diagnosis, in combination with standard tPA is safe and may improve outcome. Among 126 patients randomized to tPA plus 2-hour TCD monitoring (target group) or tPA alone (control group), symptomatic ICH occurred in 4.8% of target and 4.8% of control patients. Complete recanalization or dramatic clinical recovery at 2 hours after tPA bolus were observed in 49% of target and 29% of control patients (P=0.02). Moreover, trends toward better clinical outcomes at 24 hours and long term were noted in sonothrombolysis patients. A phase 3 of the CLOTBUST trial is planned to begin in 2006. Enhancement of enzymatic thrombolysis by US may allow testing regimens with low-dose tPA to reduce the risk of ICH.
Experimental and clinical studies have consistently demonstrated the capability of US to potentiate enzymatic thrombolysis. Administration of MBs may further accelerate the clot-dissolving effect of US by lowering the energy needed for cavitation. Moreover, administration of MBs has been shown to directly harm the clot surface, inducing penetrating forces and shear stress, which may promote high power jetting into the clot. Tachibana and Tachibana first described the effect of echo- contrast agents on clot lysis. These authors observed in an in vitro model that the combination of urokinase, low-frequency US (170 kHz), and MBs resulted in an increased rate of fibrinolysis at 60 minutes compared with urokinase plus US or urokinase alone. The bioeffect of US-mediated MB destruction on clot lysis has been demonstrated even in the absence of thrombolytic drugs, with the use of a variety of echo-contrast agents in combination with low-frequency, high-power US. Recently, in an in vitro study a high rate of declotting was observed with the combination of tPA, high-frequency commercially available US (2-MHz), and galactose-based air-filled MBs (Levovist). The present study demonstrates that the administration of galactose-based MBs during continuous 2-MHz US monitoring may influence the completeness of tPA-induced MCA recanalization. Our findings are in agreement with a recent pilot study by Viguier et al in 8 patients with proximal MCA occlusion, showing a high rate of recanalization (50%) when tPA, MB infusion, and 1-hour continuous transcranial color-coded sonography monitoring were combined. On the other hand, we observed a higher rate of sudden recanalization in the MB group (21%) than in those patients who did not received MBs, which is in consonance with experimental studies showing that destruction of MBs by US is associated with clot dissolution rather than clot fragmentation.
Although US contrast agents have been shown to be safe when given in acute stroke patients, safety information of these agents in the setting of stroke thrombolysis is limited. In the present study the administration of 3 boluses of 400 mg/mL of Levovist was well tolerated in all patients without systemic complications. Moreover, neither continuous 2-MHz US monitoring nor the combination of US plus MBs increased the rate of SICH compared with tPA alone. This is in contrast to the increased rate of SICH observed in stroke patients treated with tPA plus low-frequency (300 kHz) US. In fact, safety appeared to be better in the 2-MHz US study CLOTBUST (SICH rate 4.8%) compared with the Transcranial Low-Frequency Ultrasound-Mediated Thrombolysis in Brain Ischemia (TRUMBI) trial (SICH rate 35%), which used 300 kHz US. Unlike high-frequency US, low-frequency US application may cause mechanical distortion of the human brain microvessels, leading to vessel disruption. Daffertshofer et al have hypothesized that reverberations of the long-wavelength US inside the head may produce "hot spots" of US energy. Our observations indicate that US contrast agents may be administered safely during sonothrombolysis with high-frequency US.
Several factors may influence the effect of MBs on sonothrombolysis, including bubble size, stability of the bubble in bloodstream, and the concentration of MBs in the front of clot degradation. Room air–filled MBs are less stable than MBs filled with heavy-molecular-weight gases (eg, sulfur hexafluoride or perfluoropropane), which decrease solubility and improve bubble lifetime. Moreover, these second-generation MBs have much smaller diameters than room air–filled MBs, which improve passage of the pulmonary capillary bed. Furthermore, serial bolus administration may ensure a massive arrival of MBs striking the offensive clot compared with continuous MB infusion. Whether second-generation MBs are more effective than galactose-based air-filled MBs requires further investigation.
This study has certain limitations. Patients were not randomized to receive tPA+US+MBs and were not treated at the same period of time as the other groups. Although baseline characteristics were similar among treatment groups, imbalance cannot be ruled out because of the small sample size. Moreover, the small size precludes the assessment of the effects of MBs among stroke subtypes. Furthermore, because the present study was focused on patients with MCA occlusion, our findings should not be extrapolated to patients with stroke involving other vascular territories. Finally, in our study the assessment of recanalization by the sonographers was unblinded. Therefore, future studies should include an independent evaluation of recanalization with MR or CT angiography.
In patients with MCA occlusion combined treatment with t- PA+US+MB was associated with both early recanalization and high rate of asymptomatic hemorrhagic transformation as compared to patients treated with tPA plus US without MB. This phenomenon appears unrelated to MB administration during sonothrombolysis. Our observations using air-filled galactose-based MB are in contrast with the TUCSON trial. The Transcranial Ultrasound in Clinical Sonothrombolysis (TUCSON), a dose escalation phase II study of perflutren-lipid microspheres combined with systemic tPA and ultrasound, was prematurely stopped due to a high rate (27%) of symptomatic ICH in the second dose tier. Safety concerns of perflutren- lipid microspheres will require further experiments to determine the mechanisms how microspheres interact with tissues promoting intracranial bleeding.
Exeprimental studies using US contrast agents with air-filled albumin microbubbles have been shown to produce alterations of endothelial permeability, haemolysis and bleeding, proportionally to the intensity and duration of US exposure and the dose of contrast agent. The leading hypothesis suggested is probably the enhanced cavitational effects
The shock waves generated by bubbles explosion can induce endothelial injury: this damage mechanism is thought to be created by the collision between expanded microbubbles and the vascular wall, causing microvessel rupture and subsequent haemorrhage. However, data regarding the effect of US-activated MB on an already disrupted BBB in the course of cerebral ischemia is lacking. Recently, experimental studies showed that US neither promoted additional BBB disruption nor increased apoptosis and markers of tissue damage outside the infracted area. On the other hand, the use of potentiated IV thrombolysis by US plus MBs, enhancing the penetration of t-PA in the thrombus, could allow to lower the dose tPA, thus reducing the risk of ICH.
CEREBROLYSIN
D. Muresanu
Department of Neurology, Faculty of Health Sciences, University of Medicine and Pharmacy "Iuliu Hatieganu", Cluj-Napoca, Romania
Cerebrolysin is a combination of active fragments (polypeptides) of different neurotrophic factors, obtained by a standardized controlled breakdown of highly purified specific proteins.
The neurotrophic factors have multiple roles:
Neurotrophicity denotes a natural biological process by which the continuous effort of the cell maintains correct DNA expression, thus maintaining a normal phenotype.
Neuroprotection represents the sum of all mechanisms directed against harmful factors.
Neuroplasticity is the permanent adaptation to new functional horizons and responsibilities.
The concept describes the brain’s ability to change already-existing structures in response to environmental stimuli, such as learning, experiencing something new, or injury.
Neurogenesis is the process by which new nervous tissue cells (such as neurons, astrocytes and oligodendrocytes) are created from stem cells. In a strict sense, neurogenesis is defined as the creation of new neurons.
Neurotrophicity, neuroprotection neuroplasticity and neurogenesis are biological processes that take place continuously in the nervous system.
We cannot therapeutically control complex pathophysiological cascades with only one neuroprotective molecule, having a single mechanism of action. The future belongs to rigorously controlled drugs, having a pharmacological action as closed as possible to the biological mechanisms, with a minimal toxicity and having a pleiotropic effect on the pathological cascades, acting reversibly for a limited period without blocking the secondary reparatory neuroplasticity.
Such molecules are the neurotrophic factors and neurotrophic-like molecules which stimulates all basic biological processes therefore having a multimodal activity.
In absence of neurotrophic factors the nervous cells suffers a process of apoptosis.
The main disadvantage of the neurotrophic factors - polypeptides with heavy molecular weight - is that they do not pass the blood brain barrier.
This is not the case of the active fragments that are in the composition of Cerebrolysin.
Cerebrolysin active fragments of neurotrophic factors obtained through controlled breakdown pass the blood brain barrier and involve themselves in the neuroprotection, neuroplasticity, neurotrophicity and neurogenesis processes.
Mechanism of action: Neurotrophic factors active fragments quickly pass the blood brain barrier and bind the specific receptors on different membranes of the nervous system. Each fragment specifically initiates an intracellular signaling pathway by phosphorilation of the involved proteinkinases, finally leading to activation of the transcription factors and production of proteins involved in maintaining the cellular homeostasis and in neuroplasticity processes.
Experimentally were also proved other independent mechanisms: antioxidant, stabilizer of the blood brain barrier, aerobic neuronal metabolism stimulator, stabilizer of the P35/CDK5 molecular complex with a fundamental role in neuroprotection in neuroplasticity.
In conclusion the mechanism of action is pleiotropic, blocking several pathogenical links simultaneously in the pathological cascade in stroke.
As mentioned before, Cerebrolysin has also multimodal mechanism, being able to regulate in the same time all basic biological processes.
Multiple clinical studies suggested the efficacy of the Cerebrolysin treatment, recommending it as a potential drug in acute and neurorehabilitation therapeutical strategies.
8265 patients were included in 115 clinical trials. Of these, 2038 were acute strokes (1355 treated with Cerebrolysin and 638 with placebo).
Cerebrolysin is also a very well tolerated drug.
NORMAL VERSUS PATHOLOGICAL NEUROPLASTICITY
D. Muresanu
Department of Neurology, Faculty of Health Sciences, University of Medicine and Pharmacy "Iuliu Hatieganu", Cluj-Napoca, Romania
Neurotrophicity, neuroprotection, neuroplasticity and neurogenesis are the most important endogenous basic biological processes that act together under genetically control to generate endogenous defense activity (EDA), a polychromic continuous process of nervous system which aim to counteract the pathophysiological processes.
Neuroplasticity is the permanent adaptation to new functional horizons and responsibilities. It is the concept that describes the brain’s ability to change the already existing structures in response to environmental stimuli, such as learning or experiencing something new or to an injury.
Neurorecovery is the positive outcome of EDA producing clinically relevant results, with immediate functional and late structural effects. Immediate and late effects generate three types of changes: restitution, substitution and compensation. All basic biological processes can be activated endogenously (naturally) or exogenously.
In order to successfully compete with pathophysiological processes and support recovery, EDA effects might be enhanced by: pharmacological support, physical means, electromagnetic stimulation, psychological support, environmental stimulation, stem cell transplantation or any demonstrated combinations of these factors capable of improving patient condition.
Basic biological processes share common mechanisms with pathophysiological processes (e.g. excitotoxicity and neurotrophicity together with neuroplasticity have, as a common important driver, the NMDAR activity). In the same time inflammation has an important contribution for neuroregeneration, stimulating neuroplasticity, via trophic factors.
Regulation disturbances in each of the four major players of EDA are able to generate pathological conditions. A deficit of neurotrophicity will always increase the susceptibility for a lesion. So far we do not know any kind of pathologies with an excess of neurotrophicity or neuroprotection. For neuroplasticity both up-regulation and down- regulation generates pathologies (down-regulation generates a deficit of recovery while up-regulation generates hundreds coins of neuropathological plasticities such as neuropathic pain, multiple sclerosis, movement disorders, tinnitus and more).
The presentation will focus on some potential pathological outcomes of neuroplasticity.
THE BENEFIT OF PHARMACOLOGICAL MODULATION IN NEURORECOVERY
D. Muresanu
Department of Neurology, Faculty of Health Sciences, University of Medicine and Pharmacy "Iuliu Hatieganu", Cluj-Napoca, Romania
Endogenous defense activity (EDA) of the nervous system is a polychronic continuous process that simultaneously performs activities of neurotrophicity, neuroprotection, neuroplasticity and neurogenesis (basic biological processes).
Neurorecovery is the positive outcome producing clinically relevant results, with immediate functional and late structural effects.
Immediate and late effects generate three types of changes: restitution, substitution and compensation.
All basic biological processes can be naturally activated endogenously or exogenously.
In order to successfully compete with pathophysiological processes and support recovery, EDA effects might be enhanced by: pharmacological support, physical means, electromagnetic stimulation, psychological support, environmental stimulation, stem cell transplantation or any demonstrated combinations of these factors capable of improving patient condition.
The presentation will focus on some pharmacological strategies capable to enhance neurorecovery.
SHOULD SURGERY BE OFFERED TO PATIENTS WITH EXTRA-TEMPORAL EPILEPSY IF THE MRI DOES NOT SHOW A STRUCTURAL LESION? YES
M. Neufeld
Epilepsy Unit and EEG laboratory, Tel-Aviv Medical Center, Tel-Aviv, Israel
What support does the literature offer for this claim? Until now, there has been only one randomized long term study (of temporal lobe epilepsy), and several controlled trials (of temporal, or grouped temporal and extra- temporal lobe epilepsy) evaluating seizure outcome of epilepsy surgery versus treatment by medication. There are no controlled studies comparing extra-temporal surgery alone – whether it is lesional or MRI- negative – to no surgery. Therefore, we must approach this issue using other established literature to substantiate surgery in extra-temporal MRI -negative patients.
First of all, by virtue of continuously evolving MRI techniques which may enhance the identification of a lesion, the term "MRI negative" does not necessarily mean that a structural lesion does not exist. Furthermore, in the absence of a proven structural lesion on MRI, we have recourse to a battery of other available technologies at our disposal.
Effective use of presurgical evaluation is essential in attaining a better postsurgical outcome in patients with “non-lesional” extra- temporal epilepsy. Intracranial EEG recordings, which are required for most of these cases, rely on integrating the results of functional and non- invasive tests such as SPECT, PET, and MEG prior to implantation.
Successful ictal SPECT scans can be extremely valuable in localizing the cerebral area generating partial seizures. Findings using subtraction perictal SPECT co-registered to MRI (SISCOM) were predictive of pre-surgical outcome independent of MRI findings or scalp ictal EEG. Also, applying statistical parametric mapping (SPM) analysis to SPECT and FDG-PET may increase the sensitivity for detecting a localized abnormality.
MEG-based source localization – which is routinely combined with structural imaging – has been proven effective in measuring extra- cranial magnetic fields generated by intra-neuronal electric currents. MEG also has been shown to more easily detect spikes when they occur at lateral neocortical areas; accordingly, MEG studies in extra-temporal neocortical epilepsy have higher yields in localizing the epileptogenic zone than do studies in patients with temporal epilepsy.
The literature provides us with additional evidence to warrant the surgery option. Studies have shown that scalp recordings with a well- defined and exclusively unilateral inter-ictal epileptiform pattern over a single region as well as fast discharges at the onset of frontal seizures in non-lesional frontal lobe epilepsy were predictors of excellent surgical outcome regardless of MRI findings.
Intracranial recordings with an ictal onset pattern consisting of low voltage gamma frequency or high amplitude beta spikes for the duration of the seizure may identify patients with greater likelihood of seizure freedom following surgery.
Functional MRI localization of epileptiform spike-related blood changes and magnetic resonance spectroscopy are promising technologies which might contribute to successful surgery in patients with "non- lesional" extra-temporal epilepsy.
Over the years, studies have shown a continual improvement of long-term outcome in MRI-negative extra-temporal surgery patients. This may be attributed to the ongoing development of more precise imaging and seizure mapping such as those discussed above.
Complications associated with invasive recordings will inherently be reduced by the effective and coordinated use of these methods in discerning the focus area prior to intracranial electrode implantation. Refining the applications of these procedures defines the epileptogenic zone thereby decreasing the extent of implantation, reducing complications, increasing the success rate, and helping to predict a better postsurgical outcome in patients with "non-lesional" extra-temporal epilepsy.
In conclusion, surgery is worthwhile in patients with MRI-negative extra-temporal epilepsy when coupled with the judicious use of appropriate and conscientious pre-surgery evaluation while balancing the chances of improvement with surgery against the risk of invasive recordings and surgical complications.
THE BEST TREATMENT FOR ADVANCED, FLUCTUATING PARKINSON´S DISEASE: DUODOPA
P. Odin
Central Hospital Bremerhaven, Germany
A problem with peroral L-dopa therapy is the occurrence of fluctuations in the effect of the medication, seen in a majority of the patients, already after a few years of treatment. This depends on the correlation of dopamine concentration at the striatal dopamine receptors with the plasma L-dopa concentration. L-dopa is resorbed first in the proximal part of the small intestine and the plasma concentrations are therefore dependent on an often very irregular gastric emptying. In more advanced stage not only the plasma concentration, but also the dopamine concentration at the dopamine receptors become irregular. Improvements of the oral therapy, including the introduction of MAO-B and COMT inhibitors, have not solved this problem. Therefore methods for continuous infusion of L- dopa (intravenous or intraduodenal) have been developed. Clinical studies have demonstrated that both the plasma L-dopa concentrations and the clinical symptomatology stabilize with this type of L-dopa infusions. The therapy has proved safe and effective. L-dopa/Carbidopa gel, which can be infused intraduodenally with portable pump systems, is now since 4 years on the market in several countries. A number of clinical studies have showed that L-dopa/Carbidopa gel treatment leads to improvement in motor symptomatology when given to Parkinson patients with motor fluctuations. The time in “on” increases and the time on “off,” and time with dyskinesias decrease, compared to oral therapy. We have demonstrated that also the “on”-periods get better – UPDRS III in “best on” is better under pump compared to peroral therapy. The mean dyskinesia intensity is decreased. By performing L-dopa tests with fixed doses of L-dopa before and after 6 months of Duodopa pump therapy we have shown that there is an anti-dyskinetic effect of the pump therapy. In studies on effect on non-motor symptomatology we have demonstrated clear improvements also of non-motor symptoms. According to the Non -motor symptom scale, most non-motor symptoms did improve, at least in a proportion of the patients, when switched from optimized peroral therapy to Duodopa pump infusion. Especially the improvement of the non-motor, but also of the motor, symptoms correlates to a clear improvement of health-related quality of life. In our experience side effects of this treatment mainly relate to the establishment of the PEG and to the duodenal tubing system. With improvement of the infusion equipment, these problems have become clearly less frequent during the last years. There have been no unexpected pharmacological side effects of Duodopa itself. Compared to Apomorphine Duodopa has the advantage of a stronger anti-Parkinson effect, making it possible to give it as monotherapy in all patients. Compared to DBS Duodopa has a comparable effect on motor and maybe even superior effect on non-motor symptoms. Also, Duodopa has the advantage of not having any negative neuropsychiatric effects. Duodopa represents an important and highly effective therapeutic option for patients with advanced Parkinson's disease and motor fluctuations.
ALZHEIMER'S IS NOT A REAL DISEASE
F.C.C. Peng
Department of Neurosurgery and Neurological Institute, Taipei Veterans General Hospital, Taiwan
Background: In Peng (2003) I concluded that dementia is not a disease. At that time, I was under the impression that dementia was synonymous with AD, an equation that is still held by practitioners in neurology and neuroscientists as well as lay people. It owes such an enormous impact to the erroneous history of how the eponym got started, as it has been handed down unchallenged till now.
However, in Peng (2008), I was convinced that AD never existed, doest not exist, and will never exist, and began to advocate FD (Fischer’s Disease) to replace AD. Unfortunately, the misnomer has persisted to this date. Worse, the manner and extent to which the erroneous notion is exaggerated by politicians, e.g., Newt Gingrich, have now become a vigorous political campaign to win billions of dollars from the US Government on behalf of pharmaceutical companies purportedly to end AD in 2020.
Purpose: The purpose of this lecture is to share my earnest view with you in the hope that such a misleading campaign, so downright in the wrong direction, must be corrected to shift vigor for the search and ascertainment of the true diseases that cause dementia of varying forms which ironically have so far been lumped together as AD. My view is discussed in four parts below.
Discussion: (1) To replace AD with FD by tracing their historical facts regarding how the so-called “two hallmarks” of AD erroneously got started, when Auguste did not have just the “two hallmarks”: she had arteriosclerosis, an evenly atrophic brain, miliary foci, strange substance, DM, and decubitus angina which caused her deteriorating stupors. (2) To standardize dementia as a notion of behavioral alterations caused by any one of many brain diseases or any co-morbidities thereof, rather than a brain disease by itself synonymous with AD as heretofore being erroneously so believed. (3) To propose a new division of dementia in clinical practice, viz., dementia of vascular origin and dementia of non- vascular origin. (4) And, to tackle the various causes of dementia, which are the real brain diseases, for treatment, caring, and prevention, socially, culturally, and pharmacologically in contradistinction to normal aging which may be defined as “totality of the effects of wear and tear”..
The former includes direct causes, e.g., stroke, ischemia, Binswanger’s disease, and the like, and risk factors, e.g., DM, arteriosclerosis, hypertension and hypotension, hypercholesterolemia, decubitus angina. The latter pertains to FD, HD, PSP, SCA, PD, MS, ALS, and the like.
Either way, the underlying behavioral alterations are subserved by the resultant deterioration of the brain functions of memory and cognition. The bottom line is that, vascular or non-vascular, there is a common ground: apoptosis (neuron losses or cell deaths). In other words, dementia is not a brain disease; it is the consequence of neuron losses caused by various brain diseases, leading to the deterioration of the brain functions of memory and cognition.
However, there is a catch. That is, normal aging is also caused by apoptosis which nonetheless is not limited to neuron losses; it pertains to other body cells in smooth tissues, connective tissues, and epithelium besides neural tissues. Thus, in my view, dementia is caused by the superimposition of any real brain disease on normal aging to accelerate apoptosis as a consequence that results in sped up behavioral alterations to become more noticeable than usual, clinically to physicians or otherwise to family members..
Conclusion: 1. I shall conclude that it is time to identify or determine the varying behavioral alterations clinically to explore the multifactorial cause-effect relationships of dementia and the deterioration of the brain functions of memory and cognition which are heads and tails of the same coin, rather than cognition subsuming memory; that is, we need to differentiate the consequence of the superimposition of abnormal apoptosis by any brain disease from the on-going result of normal aging.
The task is formidable for sure. Be that as it may, the first step is to come to terms with the fact that memory deals with the past and the past in the presence, while cognition handles the goings-on of the here- and-now., rather than memory being a part of cognition, because all behaviors are memory-governed. 2. In so doing, care must be taken to avoid semantic pitfalls by creating linguistically non-sequitur terms, such as vascular dementia, frontal-temporal dementia, mixed dementia, or re- inventing the wheel, like MCI and VCI.
Blood vessels do not fire because they do not have neurotransmitters. Frontal and temporal lobes refer to the locations inside the cranium. If the neural tissues are lesioned owing to abnormal apoptosis caused by a brain disease, it is likely to spread as was evidenced by Auguste who had an evenly atrophic brain affecting also subcortical structures.
Mixed dementia should not be regarded as a static admixture of two diseases, vascular dementia and AD; rather, it should pertain to a dynamic progression of either from dementia of vascular origin to dementia of non-vascular origin or from dementia of non-vascular origin to dementia of vascular origin. In the case of Auguste, she started from dementia of vascular origin, because of her arterioslerosis, DM, and decubitus angina, to dementia of non-vascular origin to end up in presbyophrenia as a mixed dementia.
From this perspective, MCI and VCI are non-sequitur; they are re- inventions of the wheel proposed by Fischer. However, where, when, and how to draw the line between simple senile dementia and presbyophrenia remain to be worked out as they constitute a continuum with time. 3. These pitfalls derive from the wrong notion that AD is a real brain disease, when it does not exist; they also fall victim of ignoring Fischer’s dichotomy of senile dementia: 1) simple senile dementia (without glandular necrosis); and 2) downright presbyophrenia (with glandular necrosis).
However, senile dementia is not a brain disease either, any more than it is synonymous with AD. It will be of clinical significance to find out which progression of dementia, from vascular origin to non-vascular origin or the other way around, is more prevalent.
IS THERE ANY NEUROREGENERATION IN AD?
B.O. Popescu
Laboratory of Molecular Medicine, ‘Victor Babeş’ National Institute of Pathology & Department of Neurology, University Hospital Bucharest, ‘Carol Davila’ University of Medicine and Pharmacy, Bucharest, RomaniaAlzheimer’s disease (AD) is the most common type of dementia in the elderly. Its neurodegenerative character refers to a progressive synaptic loss and accumulation of abnormal proteins, such as β-amyloid and hyperphosphorylated tau, which eventually lead to massive cell loss. To treat such a condition, three concepts were issued and experimented, namely neuroprotection, neurorestoration and neuroregeneration, with the mean of halting cell death, implanting new viable nerve cells and regrowth or repair of nervous tissue, respectively. Neuroregeneration could be achieved by active synaptic plasticity or neurogenesis, both with possible compensatory effects on the functionality of the affected brain areas. One important matter to be clarified is whether the brains disturbed by AD still have some capacity for endogenous neurorepair. The methods to address this particular question are limited, since the post-mortem pathological exam can provide only the ‘final picture of a long movie’. In clinical testing, neuroregeneration is suggested by the improvement of a particular cognitive function and in functional imagery examinations it is indicated by recruitment of new brain regions during performance of a specific task. There are clinical and experimental data supporting the idea that environmental enrichment, cognitive training and physical exercise could stimulate both plasticity and neurogenesis in AD patients. In some functional imagery studies, AD patients performing different tasks have shown a greater activation of particular regions of cerebral cortex or activation of cortical areas not usually activated, as compared to healthy age-matched elderly subjects, results which suggest functional plasticity. The conservation of a potential for self-regeneration of AD brains might be a chance to study and target the intimate endogenous mechanisms able to compensate for neurodegeneration.
BRAIN RESPONSES TO NAMES AMONG MULTIPLE SCLEROSIS PATIENTS WITH PSEUDOBULBAR AFFECT (PBA) AND THE EFFECTS OF DEXTROMETHORPHAN/QUINIDINE (DM/Q)
H. Pratt, G. Haiman, A. Miller
Multiple Sclerosis & Brain Research Center, Carmel Medical Center, Evoked Potentials Laboratory, Technion - Israel Institute of Technology & Rappaport Faculty of Medicine and Research Institute, Technion Haifa, Israel
Purpose: To characterize the brain activity and associated cortical structures involved in PBA, a condition characterized by uncontrollable episodes of emotional lability in patients with multiple sclerosis (MS) and assesses the effects of DM/Q on this activity.
Methods: Behavioral responses and event related potentials (ERP) in response to subjectively significant and neutral names were recorded from 33 subjects in 3 groups: 1) MS patients with PBA (MS+PBA); 2) MS patients without PBA (MS); 3) Healthy control subjects (HC). In addition, Six MS patients with PBA before (PBA-preTx) and following (PBA-DM/Q) treatment with DM/Q, and six healthy control subjects were compared. Statistical non-parametric mapping comparisons of ERP source current density distributions between groups, as well as before and after treatment, were conducted separately for subjectively significant and for neutral stimuli.
Results: Behavioral responses showed more impulsive performance in patients with PBA. As expected, almost all ERP waveform comparisons between the MS groups and controls were significant. Source analysis indicated significantly distinct activation in MS+PBA in the vicinity of the somatosensory and motor areas in response to neutral stimuli, and at pre-motor and supplementary motor areas in response to subjectively significant stimuli. Both subjectively significant and neutral stimuli evoked higher current density in MS+PBA compared to both other groups. Treatment with DM/Q had a normalizing effect on the behavioral responses of PBA patients. ERP waveform comparisons of PBA-preTx and PBA-DM/Q with HC, for both neutral and for subjectively significant stimuli, revealed effects on early ERP components. Comparisons between PBA-preTx and controls, in response to subjectively significant stimuli, revealed both early and late effects. Source analysis comparisons between PBA-preTx to PBA-DM/Q indicated distinct activations in areas involved in emotional processing and high level and associative visual processing in response to neutral stimuli, and in areas involved in emotional, somatosensory, primary and premotor processing in response to subjectively significant stimuli. In most cases, stimuli evoked higher current density in PBA-DM/Q compared to the other groups.
Conclusions: PBA of MS patients involves cortical structures related to sensorymotor and emotional processing, in addition to overactive involvement of motor cortical areas in response to neutral stimuli. Differences in brain activity were observed between pre- and post-medication patients, and DM/Q administration resulted in normalization of behavioral and electrophysiological measures.
Significance: These results may suggest that a ‘disinhibition’ of a “gate-control”-type mechanism for emotional expression may contribute to the lower emotional expression threshold of Pseudobulbar affect, and that DM/Q may alleviate this disinhibition.
TREATMENT OF PARKINSONIAN PATIENT WITH COMPLEMENTARY MEDICINE
H. Przuntek
Ruhruniversität, Bochum, Germany
Complementary medicine means diagnosis and treatment with two different medicine systems.
We use the allopathic and the traditional Indian system (Ayurveda).
The traditional Indian system is the oldest documented medicine system of the world. The healthy person has a balanced tridosha system. Diseases are caused by a dysbalance of this system.
The Indian system differentiates between Akinesia, Rigidity dominant and Tremor dominant types of Parkinsons`disease. Ayurveda is practiced by a holistic approach. The Indian Ayurveda physicians believe that PD is a disturbance of the whole body, soul and mind.
The disease is caused by genetic dysbalance and chronic intoxication. The toxins are endogenous toxins accumulated in the gastrointestinal system and in the nasal system. Parkinson's disease starts in the gastrointestinal and nasal system.
For this reason the most important therapeutic step is the detoxification of the body and the nasal treatment (nasya). The detoxification starts with diet (vata reduction), basti and different types of detoxifying massages (abayanga, pizichil) and nasya.
In addition we perform rasayana (fraily recovery). In Europe we apply in addition the classical European PD drugs in India we can apply herbal drugs like mucuna pruriens or brahmi.
In our hospital we treat patients in whom we want to prolong the L-Dopa free phase or patients in whom the additional drug treatment does not improve the condition or provokes side effects like psychoses or dyskinesias.
It has been reported, that in early motoric stages the application of dopaminergis can be retarded by panchacarma treatment.
Conclusion: Ayurveda is a traditional medicine system which considers all the essentials of the modern western medicine (Braak et al. / Przuntek, Müller, Riederer) but in addition uses detoxifying methods and yoga. The results of the complementary treatment are remarkable.
In addition we use yoga to improve the mobility, diminish stress and give the chance for spiritual way of life.
NATALIZUMAB: EFFICACY
N. Putzki
MS Clinic, Cantonal Hospital, St. Gallen, Switzerland
Currently, natalizumab is the only licensed antibody for the treatment of RRMS. Natalizumab is an immunoglobulin G4 (IgG4) Kappa monoclonal antibody directed against α4-beta-1-integrin (Very late activation antigen-4, VLA-4), a surface molecule found on all leukocytes with exception of neutrophiles. Thus, natalizumab inhibits the interaction between VLA-4 and vascular cell adhesion molecule-1 (VCAM-1) expressed on endothelial cells. It is thought that this interaction controls leukocyte adhesion, attachment and migration across the blood brain barrier into CNS.
Based on the substantial impact of natalizumab on MRI parameters in phase II, two large phase III studies (AFFIRM and SENTINEL) were conducted. The relative risk reduction on the relapse rate was 68 percent. At two years, the cumulative probability of disability progression was 17 percent in patients receiving natalizumab compared to 29 percent in the placebo group (relative risk reduction of 42 percent). Post-hoc analyses of data from the AFFIRM study were undertaken to determine the effects of natalizumab compared with placebo on the proportion of patients who were free of disease activity over 2 years. Absence of disease activity was defined as no activity on clinical measures (no relapses and no sustained disability progression), radiological measures (no gadolinium-enhancing lesions and no new or enlarging T2- hyperintense lesions on cranial MRI), or a composite of the two. Sixty- four patients taking natalizumab and 39% of 301 taking placebo were free of clinical disease activity (p<0.0001); 342 (58%) of 593 and 42 (14%) of 296 were free of radiological disease activity (43.5%, 37.9- 49.1%, p<0.0001); and 220 (37%) of 600 and 22 (7%) of 304 were free of combined activity (29.5%, 24.7-34.3%, p<0.0001) over 2 years. The effect of natalizumab versus placebo was consistent across subgroups of patients with highly active or non-highly active disease at baseline. Overall efficacy parameter of SENTINEL resembled results from AFFIRM. Head-to-head studies to determine the relative efficacy of natalizumab in comparison with other DMTs were not undertaken. Results from phase III and data from recent phase IV studies suggested superior efficacy compared to interferon-beta or glatiramer acetate and natalizumab is effective in patients with insufficient response to other DMTs.
RITUXIMAB
N. Putzki
MS Clinic, Cantonal Hospital, St. Gallen, Switzerland
Rituximab is a chimeric intravenous mAB directed against the CD20 antigen. The CD20 antigen is present on all B cells and pre-B cells. Application of rituximab results in a depletion of CD20 positive cells. Due to the fact that rituximab was licensed by the FDA for the treatment of Hodgkin´s lymphoma (HL) several years ago, there is already substantial experience with this agent (>300.000 patients have been exposed to rituximab). Also, rituximab is used for the treatment of rheumatoid arthritis (RA), systemic lupus erithematosus and other autoimmune conditions. The treatment regimen in HL usually consists of four i.v. infusions of 375 mg/m2 body surface; in RA it is mostly used with two subsequent infusions of 1000 mg two weeks apart every six months; this protocol was also applied in MS trials.
Rituximab has initially been investigated in Neuromyelitis optica (NMO, Devic´s disease) where treatment resulted in a reduction of attacks and improvement of disability (Cree et al. 2005). Also in MS, humoral mechanisms have been increasingly recognised (pattern II according to Lassmann), thus, rituximab provides an interesting approach. Early phase I and II studies demonstrated a reduction of MRI activity and patients had fewer relapses compared to the year prior to the study (Bar-Or et al. 2008). Hauser et al. (2008) conducted a randomized, placebo-controlled trial (n=104) and found a substantial reduction of Gadolineum enhancing lesions from 3 months onwards. Also, clinical disease activity was largely reduced compared to placebo. In both studies, mainly infusion-related side effects occurred; otherwise rituximab had a favourable safety profile. It was argued that the potent reduction of inflammatory activity with rituximab is rather due to an interruption in the interplay between T cells and B cells than due to an impact on humoral mechanisms. A study in primary progressive MS did not reach its primary endpoint (unpublished data).
THE HEADACHE PIPELINE OVER THE NEXT 2 YEARS
A. Rapoport
The David Geffen School of Medicine at UCLA, Los Angeles, California, USA
There are many medications used for treating migraine, some for an acute attack and some for preventing attacks. But some patients find that nothing seems to offer optimal treatment and they either have too many headaches, or they cannot get rid of them quickly enough, or they tend to recur, or they have endure unpleasant adverse events from the treatment.
I will describe a few of the many new treatments in the pipeline for the acute treatment of migraine.
CGRP Antagonists: The CGRP antagonists' olcegepant and telcagepant have been studied for some time. Telcagepant is the first oral CGRP antagonist carefully studied and results have been published in Neurology and The Lancet. It appears to work as well as a triptan with fewer triptan side effects and virtually no constriction of blood vessels. It may be able to be used in some patients who cannot take a triptan. It is hoped that it will receive FDA approval followed by approval in other countries within the next two years.
Transdermal Patches: When sumatriptan became available as a generic in several countries, various companies began to study alternate delivery systems. A sumatriptan skin patch should be approved and released soon. It is wrapped around the arm and via an iontophoretic system pushes the active drug through the skin. It works over a 4 hour period to raise blood levels of the drug higher than a nasal spray and lower than an injection. It seems to produce very few adverse events other than some reddening of the skin when the patch is removed. It will be helpful in patients who are severely nauseated and vomiting as it will bypass the gastrointestinal tract and first pass metabolism.
Oral Inhalers: Several drugs are being studied by an oral inhalation mechanism. The older drug dihydroergotamine (DHE), which has been available for our migraine patients for over 50 years, has been carefully studied in a specially designed inhaler in a phase III trial. The results are impressive in that it begins to work in 10 minutes in some patients and has excellent 2- hour pain relief and nausea, photophobia and phonophobia-free results and well as good sustained pain relief and pain free results. Surprisingly, there are very few significant adverse events when inhaled. When given intravenously most patient vomit and have other adverse events. If DHE has to be given as in injection, it usually cannot be used at home or when a patient is out of the house, and must be used in an office or hospital setting. Although the inhaler is slightly bulky, it can be used almost anyplace. This should be a good product for acute care of migraine, especially in the patient that does not respond to a triptan or for a long lasting migraine where a triptan did not work.
Neurotoxin therapy: Botulinum toxin A Injections: Botox and other forms of botulinum toxin A have been used for years for dystonia, eye muscle weakness, wrinkles and blephaospasm. They have also been used off label for headaches. Although it appears that Botox and other forms work in many patients, there have been no carefully done trials that show its superiority to placebo thus far. According the manufacturer of Botox, they have completed two Phase III trials that show significant effect over placebo in patients with chronic migraine headache. One trial has clearly met its primary endpoint and the other did not, but did meet the endpoint that the FDA preferred. The data has neither been released nor published. If it is released at the International Headache Congress in Philadelphia in September, I will report on it during my lecture in Prague and will discuss the significance.
An NSAID approved for the treatment of migraine: The first prescription form of an NSAID was just approved for migraine by the FDA. It is diclofenac potassium in sachet form and is placed into solution in water and drunk. It works much more quickly than any other oral form of this NSAID, and has been carefully studied in moderate to severe cases of migraine in double-blind, placebo-controlled trials. It would appear to be a good alternative to a triptan in triptan naïve migraineurs, and can also be taken 2 or 3 times per week in patients who have less severe headaches a few times per week and occasional more severe migraines.
Other medications and devices may be discussed if time permits.
TEMPERATURE AND BAROMETRIC PRESSURE ARE THE MOST SIGNIFICANT CONDITIONS FOR TRIGGERING MIGRAINE HEADACHE: PRO
A. Rapoport
The David Geffen School of Medicine at UCLA, Los Angeles, California, USA
Migraine in particular, as well as many other types of headaches, cause a lot of pain and suffering, family disruption, diffuculty on the job and costly disability around the world. Migraine disrupts quality of life more than most other chronic diseases, although few become totally disabled or die from migraine. Part of the problem is that migraine hits patients in the prime of their lives, from their 20s to their 50s, when they are earning money, trying to advance in their jobs and having children.
About 12% of Western populations are affected by migraine, 18% of women and 6% of men. If we add probable migraine, as diagnosed by the International Headache Society, the percentages rise. The costs are staggering, approaching 30 billion dollars of direct and indirect disability costs in the US alone.
We have some good science about the genetics and the pathophysiology of migraine, but very little science or carefully done clinical trial information about the triggers that set off a migraine attack. Many triggers are commonly cited, such as all sorts of factors in the local weather, menstrual cycle, various foods, exercise, stress, anxiety, and trauma, visual and smell triggers, sleep factors, etc. Very few of them have been carefully studied and even though patients strongly believe that these factors affect them, nothing has been scientifically proven.
Most migraineurs believe that the weather triggers their headaches and the most frequently believed triggers are temperature, humidity, wind and barometric pressure. Many others have been mentioned, such as wind and air pollution. But no study has been totally convincing.
An excellent article appeared in Neurology this year that deserves our scrutiny:
Weather and air pollution as triggers of severe headaches
Authors: Kenneth J. Mukamal, MD Gregory A. Wellenius, ScD Helen H. Suh, ScD Murray A. Mittleman, MD, DrPH
ABSTRACT
Background: The roles of weather conditions and air pollution as triggers of headache have been inconsistent in previous, generally small studies.
Methods: We performed a case-crossover study of 7,054 patients seen in a single emergency department between May 2000 and December 2007 with a primary discharge diagnosis of headache. We compared levels of temperature, barometric pressure, humidity, fine particulate matter, black carbon, and nitrogen and sulfur dioxides during the three 24-hour periods preceding presentation with corresponding levels on the remaining occurrences of that day of the week in a given month, using local meteorologic and pollutant monitors.
Results: Higher mean ambient temperature in the 24 hours preceding hospital presentation positively and linearly increased the acute risk of headache (odds ratio [OR] for a 5°C increment 1.075; 95% confidence interval [CI], 1.021–1.033; p _ 0.006). Higher risk was observed for cases with and without a discharge diagnosis of migraine and for cases between October and March or between April and September. Lower barometric pressure also increased the risk of non-migraine cases in the 48 to 72 hours before hospitalization (OR 0.939 per 5 mm Hg; 95% CI, 0.902–0.978; p _ 0.002). Current levels of pollutants did not influence the risk of headache.
Conclusions: Higher ambient temperature and, to a lesser degree, lower barometric pressure led to a transient increase in risk of headache requiring emergency department evaluation. We did not find clear association of air pollutants with risk, but cannot exclude effects of air pollution of the magnitude previously observed for stroke and other cardiovascular events. Neurology® 2009; 72: 922–927
So we see that high temperatures and to a lesser degree low barometric pressure sent people in Boston to the Emergency Room. Air pollution did not appear significant.
I particularly liked a carefully done patient survey of the effect of the Chinook winds done in Calgary by Werner Becker M.D,’s team. It has the problems inherent in a patient survey but it did show that many patients were affected by the warm Chinook winds coming off the Rocky Mountains. Some had headaches on the day before the winds blew and others on the day of the winds. They found 88% of the patients were affected by the winds but their rigorous diary collection methods only showed it to be true in 20%.
I would like to detail a carefully performed diary study by a medical student at Yale University Medical Center in New Haven, CT. She did this work along with me at The New England Center for Headache in Stamford, CT. It was published after her graduation.
The Effect of Weather on Headache
Authors: Patricia Prince, M.D., Alan Rapoport, M.D., Fred Sheftell, M.D., Stewart Tepper, M.D., Marcelo Bigal, M.D., PhD
ABSTRACT
Objectives: 1 – To assess headache patient’s beliefs about how strongly weather affects their headaches; 2 – To objectively investigate the influence of multiple weather variables on headache. Design/Methods: Our sample consisted of 77 migraineurs seen on a headache clinic which provided headache calendars for a period ranging from 2 to 24 months. Our study was divided in two phases. First, each patient was given a questionnaire assessing their beliefs about how strongly (if so) weather affected their headaches. Second, weather data were collected from the National Weather Service, from three reporting stations central to the residences of the study participants. Analysis was performed on 43 variables to generate three meteorological factors. Linear regression was used to assess the relationship between headache and these three factors. Factor 1 represents a function of absolute temperature and humidity. Factor 2 represents a changing weather pattern. Factor 3 represents barometric pressure. Results: Of the 77 subjects in the study, 39 (50.6%), were found to be sensitive to weather, but 48 (62.3%) thought they were sensitive to weather conditions (p<0.05). Thirty (38.9%) were sensitive to one weather factor and 9 (11.7%) to two factors. Twenty-six (33.7%) were sensitive to factor 1; 11 (14.3%) to factor 2; 10 (12.9%) to factor 3. Conclusions: Our study supports the influence of weather variables on headache. We showed that patients are susceptible to multiple weather variables and that more patients thought weather was a trigger than was the case. Headache 2004; 94:596-602
The study showed that 50.6% of the patients we studied were affected by weather, although an even higher percentage thought they were sensitive to weather. They often could not tell which weather factor they were sensitive to. It also showed that what weather factors the patients were sensitive to were complex but tended to be: extremes of temperature and humidity, low barometer and changes in weather factors.
Many other studies have been done including an elegant scientific study on dust as a trigger authored in part by my debate partner Professor Bolay:
African dust-laden atmospheric conditions activate the trigeminovascular system.
Authors: Doganay H, Akcali D, Goktaş T, Çaglar K, Erbas D, Saydam C & Bolay H.
Cephalalgia 2009. London. ISSN 0333-1024
ABSTRACT
It has been recently noticed that dust originating from deserts can be transported to other continents by the atmosphere and has an adverse effect on public health, such as increased asthma attacks. Dust originating from the Saharan Desert could initiate a series of reactions upon contact with cloud water and results in the formation of reduced iron (Fe2+), oxalate and various basic amino acids. We aimed to evaluate whether the simulation of Saharan dust-containing atmospheric conditions could trigger the trigeminovascular system. Freely moving rats incubated within simulated atmospheric conditions containing (I) Saharan dust, (ii) Co60 gamma ray-treated Saharan dust (sterilized) and (iii) dust-free air, were investigated for the presence of c-fos expression in trigeminal nucleus caudalis (TNC) and for NOx (nitrate+nitrite) levels in blood samples. Atmospheric samples were analyzed for microorganisms. Saharan dust- containing atmospheric conditions induced c-fos expression in nociceptive neurons within TNC. The number of c-fos+ neurons in superficial lamina of TNC was significantly higher in the Saharan dust group (32.9 ± 5.3, P = 0.0001) compared with dust-free air (11.02 ± 2.7) or Co60-treated Saharan dust groups (15.01 ± 2.4). An increase in NOx levels was detected in blood samples of rats exposed to Saharan dust- containing atmosphere. This study has revealed an unknown environmental factor as a possible trigger for headache. It is the first time that transport of Saharan dust with the atmospheric air stream has been documented to be able to trigger the trigeminovascular system in animals. Further studies are needed to explore the mechanisms and molecules that mediate the nociceptive effect and to guide new treatment strategies.
This shows how Saharan dust can affect the trigeminovascular system in animals and maybe man.
In summary, when one looks at all studies published on the weather as a trigger for migraine, temperature and barometric pressure seems to be the most frequent and definitive ones cited.
AYURVEDA MEDICINE IS EFFICACIOUS THERAPY IN PD: CON
O. Rascol
Department of Clinical Pharmacology and Neurosciences, and Clinical Investigation Center, INSERM CIC-9302, University Hospital, Toulouse, France
Ayurveda medication uses a concoction of several spices, herbs and minerals, including Mucuna pruriens that contains levodopa. This is compatible with the concept that such a traditional medicine might have antiparkinsonian properties to treat patients with Parkinson’s disease (PD).
However, the objective published available clinical data is scarce to support this assumption. We only found few trials in the English literature assessing Ayrveda medication or various formulations of Mucana pruriens in PD patients (J Altern Complement Med 1995; Nagashayana et al, 2000; Katzenschlager et al, 2004). Such studies have important methodological limitations including unblind uncontrolled design (while the placebo effect is large in PD), unconventional efficacy parameters, small number of patients, short-term follow-up, incomplete adverse events report… Therefore, the level of evidence to support the efficacy of this therapy in PD is low, mainly based on empirical experience, pathophysiological concepts and opinion leaders’ claim. The absence of evidence is not however synonymous of lack of evidence, but further investigations are mandatory before accepting such medicines as “efficacious” therapies for PD.
Moreover, the exact amount of levodopa present in such artisanal preparations is not well known and reliable pharmacokinetic and quality control data are lacking.
Finally, in spite of their generally assumed harmlessness, it is important to emphasize that traditional herbal medicines have proven to be able to induce severe adverse drug reactions. For example, 2 herbal medicines used to facilitate weight loss, namely germander (Teucrium chamaedrys) and aristolochia fangchi induced severe cases of fatal hepatitis or irreversible renal failure (Larrey et al, 1992; Cosyns, 2003).
EBM SHOULD BE THE GOLD STANDARD FOR CLINICAL DECISIONS
O. Rascol
Department of Clinical Pharmacology and Neurosciences, and Clinical Investigation Center, INSERM CIC-9302, University Hospital, Toulouse, France
There are 2 types of knowledge: implicit/subjective (tacit background, personal individual experience, intuition…) and explicit/objective (evidence, research data put in databases…). There are countless examples in Medicine of subjective empirical non evidence-based practice leading to major erroneous diagnostic procedures or pharmacological/non- pharmacological interventions (including for example surgical operations like arthroscopic surgery to relieve pain from arthritis of the knee, internal mammary artery ligation for the treatment of angina, extra-intracranial « by-pass » for the treatment of internal carotid artery occlusion…). Such prescriptions exposed millions of patients to inefficacious, toxic and expensive procedures, in the context of growing concerns about opinion leaders’ independence and potential conflicts of interest. It is therefore indispensable to propose alternative and more objective approaches.
Evidence Based Medicine (EBM) is a method for evaluating the validity of research in clinical Medicine, and applying the results to the care of individual patients. EBM systematically assesses the available (published) data and hierarchies the robustness of the corresponding evidence. EBM considers individual Randomized Controlled Trials (RCT) and systematic reviews of RCTs as the most robust evidence for assessing treatment interventions, but in the absence of such information (a very common situation), EBM also takes into account data with lower level of evidence (individual case series, case-control studies or expert consensus) to summarize the best available evidence to improve clinical decisions. Most physicians having limited time and methodological knowledge to practice individual EBM search and analysis but can efficiently use the evidence systematised by experts in unbiased reviews (ready to use) (such as Cochrane Library, MDS EBM Review) or EBM « Guidelines ».
WHICH IS THE BEST DRUG FOR EARLY PD PATIENTS: DOPAMINE AGONISTS
O. Rascol
Department of Clinical Pharmacology and Neurosciences, and Clinical Investigation Center, INSERM CIC-9302, University Hospital, Toulouse, France
There are 3 main advantages of non-ergot dopamine agonists over MAO-B inhibitors for early PD therapy:
- Agonists have a greater symptomatic efficacy on motor symptoms, as estimated with UPDRS in placebo-controlled randomized trials, although direct head-to-head comparisons are lacking. Improvement occurs rapidly, within 2 weeks, as demonstrated with novel ER formulations.
- There is robust and consistent level I evidence that the early use of dopamine agonists is associated with a reduced risk of emergence of long-term motor complications (especially dyskinesia), even when levodopa is subsequently adjunct, while the contrary has been reported with selegiline and no data are available with rasagiline
- Dopamine agonists also have positive effects on non-motor symptoms, including depressive ones, as demonstrated in a recent placebo-controlled study with pramipexole. Such evidence is lacking with MAO-B inhibitors
Like MAO-B inhibitors ER formulations of dopamine agonists can be administered once daily. Past slow and complex titration associated with the use of first generation dopamine agonists has now moved to more rapid and simple titration, especially with ER formulations. Dopamine agonists exhibit “neuroprotective” properties in in vitro and in vivo animal models of PD, and their use is associated with indirect indices (neuro-imaging biomarkers) of reduced disease progression in PD patients.
BIOBANKS CAN MAKE OR BREAK BIO MARKERS IN NEUROLOGICAL DISORDERS
R. Ravid
Netherlands Institute for Neurosciences BrainBank Consultants, Meibergdreef, Amsterdam, The Netherlands
Biomarkers are laboratory measurements reflecting the activity of a disease process; they also provide valuable information on the underlying disease mechanisms and have a potential predictive value for the progression of disease.
Biomarkers in blood, CSF and urine provide a useful aid to the clinical diagnosis as evident among others from the recent review paper on Biobanks for Biomarkers in neurological disorders (Ravid 2009).
The combination of these biomarkers, together with the risk factor ApoE έ4 genotype, seems to be the most sensitive and specific to distinguish patients diagnosed with clinical symptoms of neurodegeneration of the AD-type from those with other dementias. An accurate and early clinical diagnosis is important, because therapy should be started before extensive brain damage has occurred.
Specifically in the early stages of the disease, clinical diagnostic methods are less accurate and biomarkers supporting the diagnosis are urgently needed.
If the assumption that A42 and (hyperphosphorylated) tau in CSF obtained by lumbar puncture correctly reflect the ongoing neurodegenerative process, the concentration of these biomarkers in ventricular CSF should even the more reflect the state of the disease and the measure of neuronal damage. In contrast to biomarkers in blood, CSF biomarkers are already used by some in the diagnosis of AD and are certainly closer than blood markers to being of genuine clinical usefulness. CSF levels of hyperphosphorylated tau and Aβ 1-42 have been predictive of conversion from MCI to AD.
Currently, numerous discussions are going on concerning the advances in biomarkers research and how they can be best applied in drug discovery. One central debate issue is the Biomarker validation methodologies; this depends on several factors:, such as the size of the screened population to identify the markers, the number of times the correlation between disease and Biomarker has been made , the predictive value of a certain Biomarker and the exact correlation between the Biomarker and the disease.
While the importance of biobanks is widely recognized, the development of biobanks is still faced with many ethical, legal, social, scientific, financial, intellectual property (IP), and information technology (IT) challenges. Scientific research using clinical samples (DNA, RNA, proteins, cells, tissue, Blood, CSF and urine) resulted in an overflow of information.
There is growing demand for clinical samples to apply and implement the recently developed "-omics" technologies. By supplying biological clinically well documented specimens for research, biobanks are a major contribution to accelerating medical discoveries and underlying disease mechanisms.
The use of biomarkers to predict progression to AD in patients with MCI would yield substantial therapeutic and health-economic benefits; Differential gene expression patterns in blood cells can predict early Parkinson’s disease and possibly AD.
Focusing on signalling proteins rather than on the entire plasma proteome it is possible to identify an Alzheimer biomarker phenotype that can potentially be used for the diagnosis of AD. Intercellular communication may be an attractive target for unbiased specific screens of disease.
Comparative genomic and proteomic studies analyze the differences between diseased and normal samples to identify disease biomarkers or potential drug targets. In addition, in order to ensure an unbiased research study, organizations typically collect samples from an ethnically diverse population across a distribution of age and gender. Collection procedures differ among organizations, depending on sample type, storage requirements but all collections are performed according to validated protocols and standard operating procedures (Ravid 2008).
Biomarker assay development and validation are still under development; proper assay design will prevent later pitfalls and will facilitate the shift from preclinical to clinical diagnostics. The advent of molecular testing technologies has enabled better links between discovery of novel biomarkers and the practical application in diagnostic assays. Within the diagnostic and pharmaceutical industry there is continuous need for new diagnostic markers and biomarkers, which have an improved sensitivity and specificity.
The use of specimens obtained from large autopsy series collected and stored by Bio banks and Bio repositories, has a large number of pitfalls as previously published.
Biobanks and Biorepositories have become a vital resource to help advance our understanding of disease mechanisms. Biobanks are an essential partner in project development and plays a critical role in the speed of development and discovery; Biobanks are linked to the quality of the findings and a biorepository supplying high quality specimens can accelerate research by several years and an unfortunate choice can have the opposite effect. The main criteria to assess the suitability of the specimens offered by a Biobank are a clear code of conduct which secures the ethical and legal background of the collection, specimen integrity, and data quality.
The search for bio markers obtained from living donors has contributed already a vast amount of data. The role of amyloid and Tau as early diagnostic markers in the pathology of dementia has been reported in differential involvement in Alzheimer’s disease (AD), late onset Alzheimer disease (LOAD), Lewy Body dementia (DLBD), Vascular dementia , fronto-temporal lobar degeneration (FTLD) , Mild Cognitive Impairment (MCI) and non neurological controls.
In the coming decennia, Brain /Tissue /Bio banks (BTB-banks) will have a major role in identifying the relevant bio markers and will collect, preserve and type RNA and DNA extracted from brain /tissue /body fluids in order to update the pathological hallmarks of dementing disorders.
Biobanks and biorepositories are essential to secure and facilitate biomarker development in the future by establishing well documented collections of biological specimens as an adjunct to current research in dementing disorders.
References: Ravid, R, ‘’ Biobanks for biomarkers in neurological disorders The Da Vinci bridge for optimal clinico-pathological connection.’’. J.Neurol Sci.15; 283(1-2):119-26, 2009. Ravid R – Standard operating procedures, ethical and legal regulations in BTB (brain/tissue/bio) banking: what is still missing? Cell Tissue Bank; 9 (2):121-37, 2008. Ravid R, Grinberg LT. How to run a brain bank- revisited. Cell Tissue Bank. 9(3):149-50, 2008
SHOULD WE START TREATMENT WITH MAO-B INHIBITORS OR WITH DOPAMINE AGONIST: RASAGILINE!
H. Reichmann
Department of Neurology, University of Dresden, Dresden, Germany
Initial treatment of Parkinson’s disease (PD) should be both effective and well tolerated. In addition, as a de novo PD patient may still be working, medication should ideally be administered only once a day.
A fourth prerequisite for high compliance with treatment would be that the initial drug stops or prolongs the course of the disease and the increasing impairment of activities of daily living. In my view, all these demands are best met by rasagiline, a second generation MAO-B inhibitor. Rasagiline is given only once per day, it shows almost no side effects and improves all typical core symptoms such as bradykinesia, rigidity and tremor. There are two studies, TEMPO and ADAGIO, which suggest that this drug may have a disease modifying effect. Both studies showed that patients who are treated early will have better motor skills than those in whom drug therapy was delayed for 6 and 9 months. Therefore, rasagiline may be an ideal first-line drug for treatment of patients with PD.
MCI IN PD: MILD COGNITIVE IMPAIRMENT EXISTS IN PARKINSON’S DISEASE
I. Rektorova
First Department of Neurology, Masaryk University and St. Anne’s Hospital, Brno, Czech Republic
The incidence of dementia in Parkinson’s disease (PDD) is increased six-fold compared to the general population, and PD patients have cognitive deficits, in particular executive and attentional dysfunction, even in the absence of frank dementia. Mild cognitive impairment (MCI) was first characterized by Petersen as a transitional state between the cognitive changes of normal aging and dementia. Amnestic MCI is the most common one and refers to a memory loss that is more pronounced than one would expect for age, yet the patient does not meet currently accepted criteria for clinically probable Alzheimer’s disease (AD). Most of these subjects will progress to AD at a rate of 10-15% per year, compared to the 1-2% conversion rate in healthy controls. But both amnestic and non -amnestic MCI have been identified with a single or multiple domains impaired and with a potential risk for progression to different types of dementia.
It has been demonstrated that mild cognitive impairment in PD (MCI_PD) exists between cognitively normal PD and PDD, and it may be defined by applying criteria similar to the MCI that is posited as a precursor of AD. MCI_PD seems to be under-diagnosed in clinical practice due to routine use of insensitive screening instruments such Mini-Mental State Test (MMSE). Results of a recent study demonstrated a twofold increase in the proportion of MCI in subjects with early, untreated PD compared to controls. Patients may have both non-amnestic (more common) and amnestic types of cognitive impairment. Various biomarkers (and brain imaging in particular) hold promise in indentifying and longitudinal follow-up of MCI_PD patients. Preliminary data have shown that patients with MCI_PD had a higher risk of developing dementia than cognitively intact PD patients. Larger follow-up studies in MCI_PD cohorts will help to assess the sensitivity and specificity of the PD-MCI designation as far as dementia development is concerned.
In conclusion, identification of MCI in PD is important, as it offers an opportunity for further study of cognitive impairment in PD, and may eventually be a target for pharmacological intervention to prevent or delay the development of dementia.
Supported by grant MSD 0021-622-404 from the Czech Ministry of Education
INTRAVENOUS IMMUNOGLOBULIN IS AN EFFECTIVE TREATMENT FOR ALZHEIMER’S DISEASE: PRO
N. Relkin
Weill Cornell Medical College, Gammaglobulin Alzheimer Partnership (GAP) Study, New York, USA
Since the late 1990s, there has been increasing evidence that immunotherapy targeting beta amyloid (Aβ) can be used to treat Alzheimer’s disease (AD). Several clinical trials are in progress using active vaccination or synthetically modified murine antibodies targeting linear epitopes of the Aβ molecule. Intravenous immunoglobulin (IVIG) is distinctive in that it contains natural human antibodies against conformational epitopes of Aβ aggregates, including oligomer and fibrils. These antibodies in IVIG bind with moderate affinity to neoepitopes formed by a variety of misfolded amyloid proteins, altering their aggregation properties and exerting immunoomodulatory influences on their clearance by microglia. In a number of human studies carried out to date, IVIG infusions altered Aβ levels in plasma and CSF in a dose-dependent fashion and yielded positive effects on cognition and global function. In addition, IVIG treatment increased cerebral metabolism in Alzheimer-vulnerable areas of the brain. These results, coupled with recent positive epidemiologic findings and IVIG’s established record of safe use for more than 25 years as an immune replacement therapy, provide a strong rationale for IVIg’s development as an investigational treatment for AD. Its limited supply and high cost notwithstanding, IVIG can help initiate a new generation of disease modifying treatments if the ongoing Phase 3 study confirms its safety and effectiveness in patients with AD.
Disclosure: Dr Relkin has received grants for research on IVIG’s use in Alzheimer’s from the US National Institute of Aging and Baxter Inc.
References: Dodel, R, Du Y, et al Intravenous immunoglobulins containing antibodies against a-amyloid for the treatment of Alzheimer’s disease, JNNP, 2004. Weksler ME, Relkin N, Turkenich R, eta al. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol 2002. Relkin N, Szabo P, Adamiak B, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2009.
STATINS SHOULD BE GIVEN TO EVERY NON-CARDIOEMBOLIC ISCHEMIC STROKE PATIENT FOR SECONDARY STROKE PREVENTION
D. Russell
Norway
Stroke is estimated to affect 10 million people worldwide every year. Patients who have had a stroke or a transient ischemic attack (TIA) are at high risk for recurrent cerebrovascular and cardiac events. Regardless of stroke subtype, the prevalence of coronary atherosclerosis in patients with stroke is 75%. After a first stroke, the 5-year risk of having another stroke is 20% and the 5-year risk of myocardial infarction is 10%, which qualifies stroke as a coronary heart disease risk equivalent (i.e. a 10-year risk of myocardial infarction of 20%).The occurrence of stroke increases with age, especially the elderly, a population who also have a high risk for coronary heart disease. This knowledge in itself may be a sufficiently compelling to consider statin therapy in all of these patients.
Statin trials have included over 90 000 patients to determine the effect of statins on the incidence of major cardiovascular events in patients at high vascular risk. In these trials, stroke was a secondary endpoint. The relative risk reduction for stroke was 21% [odds ratio (OR) 0.79 (0.73–0.85)] with no heterogeneity between trials. Fatal strokes were reduced, but not significantly, by 9% [OR 0.91 (0.76–1.10)]. The extent of the statin effect was closely associated with low-density lipoprotein-cholesterol (LDL-C) reduction. LDL reduction explained 34 –80% of the observed benefit, leaving the possibility for other, pleiotropic effects. Each 10% reduction in LDL-C was estimated to reduce the risk of all strokes by 13.2% [95% confidence interval (CI) 4.8–20.6] and carotid intima-media thickness by 0.73% per year (95% CI 0.27–1.19). A meta-analysis of these studies showed that statins may reduce the incidence of all strokes, and this effect was mainly driven by the extent of between groups LDL-C reduction. Another meta-analysis, using individual data of 90 000 individuals, came to the same conclusion. It showed a reduction in fatal and nonfatal stroke [relative risk (RR) 0.83, 0.78–0.88, P<0.0001] and that statin therapy can reduce the 5-year incidence of major coronary events, coronary revascularization and stroke by about one-fifth per mmol/l reduction in LDL cholesterol This was largely irrespective of the initial lipid profile or other presenting characteristics There was also a good overall safety profile with no increased incidence of hemorrhagic stroke and cancer.
The heart protection study (HPS) showed that treatment with simvastatin (40 mg) in patients with TIA or stroke led to reductions in the risk of cardiac events but not of recurrent stroke. The main explanation for this neutral effect was pobably the fact that the study was not powered for this comparison, and that patients had their qualifying stroke on average 4.3 months prior to randomization, at a time where the risk of stroke has naturally decreased to its lowest level. The risk of myocardial infarction, on the other hand, continuously increases over time after a stroke or TIA.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) study was dedicated to stroke patients. This large, randomized, placebo-controlled trial evaluated atorvastatin 80 mg/day in patients with stroke or TIA. In this study, patients with recent stroke or TIA within 1–6 months of study entry and LDL cholesterol of 100– 190mg/dl without known coronary heart disease (n=4731) were randomly assigned to double-blind treatment with atorvastatin 80 mg/day or placebo. The primary endpoint was occurrence of first fatal or nonfatal stroke. During the trial, mean LDL-C was 73 mg/dl on atorvastatin and 129 mg/dl on placebo. After a median follow-up of 4.9 years, a fatal or nonfatal stroke occurred in 265 patients (11.2%) receiving atorvastatin and 311 patients (13.1%) receiving placebo (5-year absolute risk reduction (RR) 2.2%; adjusted hazard ratio (HR) 0.84, 95% CI 0.71– 0.99, P= 0.03). Two hundred and eighteen ischemic and 55 hemorrhagic strokes occurred with atorvastatin and 274 ischemic and 33 hemorrhagic strokes with placebo. The absolute 5-year risk reduction in major cardiovascular events was 3.5% (HR 0.80, 95% CI 0.69–0.92, P¼ 0.002). Overall mortality was unchanged (216 vs. 211 deaths for atorvastatin and placebo respectively, P=0.98). The rates of serious adverse events such as muscle pain, myopathy and rhabdomyolysis were similar. The SPARCL trial has therefore shown that in patients with recent stroke or TIA without known coronary heart disease, a 5-year treatment with atorvastatin 80 mg/day reduced the incidence of stroke and cardiovascular events .This result was obtained despite poor adherence to the allocated randomized treatment, particularly in the placebo group. On average, 25% of patients in the placebo group were prescribed a commercially-available statin outside the trial. In a post-hoc analysis, LDL-C reduction was used as the best marker for adherence to the allocated treatment with the hypothesis that patients with no change or an increase in LDL-C (vs. baseline) were not on statin or adherent to allocated placebo, while the group with over 50% LDL-C reduction from baseline were likely adherent to atorvastatin 80 mg/day. Based on 55 045 blinded LDL-C measurements (with an average 11.6 measurements per patient performed during the follow-up), percentage change in LDL-C from baseline was classified post hoc as no change from baseline, under 50% reduction or at least 50% reduction. Compared with the group with no change or an increase in LDL-C, the group with the greatest LDL-C reduction (>50% from baseline) had a 31% relative risk reduction in stroke and no increase in brain haemorrhage.
In conclusion statins are effective in reducing the risk of stroke in populations of patients at high vascular risk, as well as the risk of major coronary events. In secondary prevention of stroke, statins clearly reduced the risk of a major coronary event and the SPARCL trial has shown that atorvastatin reduced the risk of recurrent stroke. The European Stroke Organization's 2008 guidelines recommend statin therapy in subjects with non-cardioembolic stroke (Class I, Level A).
WHICH IS THE BEST DRUG FOR EARLY PD PATIENTS? LEVODOPA
E. Ruzicka
Department of Neurology, Charles University in Prague, Czech Republic
The introduction of levodopa more than 40 years ago dramatically improved life expectancy of Parkinson’s disease (PD) patients and reduced their morbidity due to medical complications of progressive immobility. Compared with all other symptomatic drugs, levodopa has the greatest efficacy in reducing motor symptoms of PD. In comparison with direct acting dopamine agonist drugs, levodopa is faster in achieving therapeutic dose and is less likely to induce psychosis, daytime somnolence, behavioral addiction, impulse control disorder, cardiac valvulopathy or leg edema. Last but not least, levodopa is substantially less expensive than dopamine agonists.
Putative neurotoxic effects of levodopa through enhanced oxidative stress have never been confirmed in clinical practice and in the contrary; there is fair evidence of neuroprotective effects of levodopa therapy. On the other hand, dyskinesias are typically associated with levodopa therapy, especially in young-onset PD patients. However, these dyskinesias are usually mild and non-disabling, often even going unnoticed by the patient. In the long term (after more than 5 years of PD duration), clinical outcomes are generally equivalent in patients initially treated with either levodopa or a dopamine agonist.
In conclusion, after four decades of clinical experience, levodopa remains the „gold standard“ in PD therapy and it should be used as the treatment of first choice, except perhaps in patients with young onset PD at high risk of developing early dyskinesias.
ROPINIROLE PROLONGED RELEASE
E. Ruzicka
Department of Neurology, Charles University in Prague, Czech Republic
Ropinirole is a non-ergot dopamine D2/D3 receptor agonist indicated for the treatment of Parkinson's disease (PD) and restless legs syndrome (RLS). As monotherapy in early PD, ropinirole improves signs and symptoms of the disease and leads to a lower incidence of dyskinesias compared with initial treatment with L-DOPA. In addition, an 18F-DOPA PET study suggested that ropinirole could slow the progression of loss of dopamine neurons compared with treatment with L-DOPA. As an adjunct to L-DOPA in advanced PD patients with motor fluctuations, ropinirole reduces off time and allows a reduction of L-DOPA dose.
Ropinirole prolonged release (PR) is a novel once-daily, 24-hour formulation of ropinirole. It has been approved as monotherapy and as an adjunct to L-DOPA in the treatment of PD. Potential advantages of ropinirole PR compared to the immediate release (IR) formulation include maintaining more consistent dopaminergic activity with steadier plasma levels, increased tolerability, greater compliance with a once-daily dosing regimen and ease in dose titration. In early PD patients, a similar efficacy and tolerability but significantly greater compliance of ropinirole PR has been shown in comparison to IR formulation. In advanced PD, daily “off” time was reduced with ropinirole PR compared to placebo and to ropinirole IR. In addition, sleep quality and nighttime motor symptoms appear to be improved with ropinirole PR in patients with more severe sleep-related problems.
Overnight switch with an approximate 1:1 conversion ratio from ropinirole IR to PR was well tolerated and occurred without loss of efficacy. The adverse effects of ropinirole PR are similar to other non- ergot dopamine agonists, including nausea, somnolence, edema, orthostatic hypotension, hallucinations and dyskinesias.
In conclusion, ropinirole PR is a safe, well-tolerated option for treating early and advanced PD. Results of studies using ropinirole PR in RLS are to be expected.
IV TPA BEYOND 3 HOURS: IS IT SUITABLE FOR EVERYONE? NO
P. Sandercock
UK
The approval for the use of IV rt-PA in the EU is very clear and also quite restrictive. The current license states that treatment is indicated for patients who are aged under 80 years and who: have a definite ischemic stroke; can start treatment within 3 hours of onset; do not have a history of prior stroke + Diabetes; are not at excessive risk of bleeding (there is a long list of conditions which preclude the use of iv thrombolysis for either stroke or myocardial infarction); have a stroke deficit that is not ‘too mild’ (NIHSS < 4) or too severe (NIHSS > 25) and do not have ‘extensive infarction’ on CT.
The ECASS-3 trial evaluated the effects of IV rt-PA 3-4.5 hours after onset in people who otherwise met all the above criteria. The manufacturers of rt-PA have submitted applications to the FDA and the EMEA to extend the approval for use in these very carefully selected groups of patients, to 4.5 hours, but at the time of writing, approval is still pending.
The current use of IV thrombolytic treatment is still limited. In population-based studies, the overall proportion of ischemic strokes who receive treatment is 6.3% in Sweden, 4.0% in Germany, 2.4% in USA and 1% in UK. There are a number of factors behind this limited use, the most important of which are the many clinical exclusion criteria and age >80. The EU license is restricted to patients aged < 80, since there are essentially no randomized data on the balance of risk and benefit in this age group. Partly because of the EU license, but also because of concerns about efficacy and safety in older people, thrombolysis treatment rates have been shown to decline steeply with age in many of the countries where there are reliable data. In the UK, for example, about 30% of all strokes are aged > 80, so about 31,000 ischemic stroke patients each year are automatically excluded from thrombolysis by virtue of their age alone. Mild or rapidly improving strokes (NIHSS < 4) are another category in whom treatment is not approved, yet a significant proportion may suddenly deteriorate later, and end with significant disability.
Although some guidelines are recommending use of iv rt-PA after 3 hours, it is worth examining the evidence from the Cochrane systematic review of the randomized trials of iv rt-PA treatment up to 6 hours which includes the ECASS-3 & EPITHET data. In total, there have been 11 trials, which included 3977 patients, only 42 of whom were aged > 80 years. The effect of i.v. rt-PA < 6h on death at the end of follow-up was a non significant trend to a 14% increase in the odds of death (95% CI 5% reduction to a 38% increase). There was a consistent excess of fatal intracranial hemorrhage across all the trials, with an overall absolute risk of about 3%. Despite these risks, the effect of i.v. rt-PA < 6h was a significant 22% reduction in the odds of being ‘dead or dependent’ (mRS 3-6) (95% CI 12-32%). However, the estimate of effect was difficult to interpret since it was associated with substantial heterogeneity (Chi2 p = 0.007) I2 = 62%.
Whilst these data suggest that benefit to at least six hours is plausible, it is not clear which categories of patients will benefit most and which are most likely to be harmed. Furthermore, there is still no reliable randomized evidence on risks and benefits in patients aged over 80. Hence one cannot yet make a recommendation for thrombolysis after 3 hours ‘for all’. Indeed, further large-scale randomized trials comparing thrombolytic therapy with control in patients with acute ischemic stroke who do not exactly match the current license criteria, in whom IV rt-pa is ‘promising but unproven’ are still needed. IST-3 is such a trial, and it seeks to determine whether a wider variety of patients may benefit from IV therapy; it will report its results in early 2012.
SHOULD MECHANICAL EMBOLECTOMY DEVICES BE USED IN ROUTINE CLINICAL PRACTICE? NO
P. Sandercock
UK
Reperfusion therapy seeks to restore blood flow after an ischemic stroke due to occlusion of a cerebral artery. There is good evidence that prompt thrombolytic therapy with intravenous rt-PA is effective in appropriately selected patients aged less than 80 years who can be treated within 3 hours. Recent evidence suggests that the time window for IV treatment may extend to 4.5 hours for selected patients. No thrombolytic agent has yet been approved for intra-arterial use in stroke.
A variety of devices are now also available which can retrieve thrombi from occluded cerebral arteries. However, the regulatory approval system for devices is different for that for drugs. For devices, one only needs to establish that the device can retrieve the clot from the artery. This is a distinct contrast with the approval process for drugs. For devices, there is no regulatory requirement for randomized controlled trials to establish that use of the device in specific types of patients will result in net clinical benefit. ‘Net clinical benefit’ is usually taken to mean ‘used in routine practice, this device does more good than harm.’
For patients with acute coronary syndromes, large scale randomized trials first established the value of IV thrombolysis. These trials were then followed by large scale trials of primary coronary stenting versus IV thrombolysis which established the superiority of primary stenting over IV thrombolysis.
The situation is quite different in acute stroke. To date, the only evidence on the clinical effectiveness of embolectomy in stroke has come from small uncontrolled case series. These suggest that mechanical clot retrieval therapy might be effective in highly selected patients treated in very well-resourced super-specialized acute stroke centers, but that safety is unclear. So, for clot retrieval devices, although several are ‘approved,’ there is – so far – no reliable evidence from randomized controlled trials that compared with established therapies, mechanical clot retrieval does more good than harm.
However, some randomized controlled trials in stroke are under way. The IMS-3 trial seeks to recruit 900 patients to determine whether a combined IV/IA approach to recanalization is superior to standard IV rt- PA alone when initiated within 3 hours of stroke onset. The MR RESCUE trial seeks to recruit 120 patients to determine within 8 hours of onset if diffusion-perfusion MRI can identify patients who might benefit from mechanical embolectomy with a balloon catheter and retriever. These trials should help provide estimates of the balance of risk and benefit and the correct patient selection criteria for the procedure (though not its cost-effectiveness. In the USA, medical insurance companies provide very substantial financial reimbursement for the use of mechanical embolectomy devices in acute stroke (US$ 23,000 per procedure) whereas the Diagnosis-Related Group reimbursement for iv thrombolysis is only $11-12,000. Thus, a non-evidence-based therapy attracts a very much higher reimbursement than standard therapy. This appears to be a significant impediment to recruitment in the ongoing trials.
However, until the RCT’s have been completed, mechanical embolectomy should not be used in routine clinical practice – despite the perverse financial incentives - and should only be used in the context of properly conducted randomized controlled trials.
EARLY TREATMENT FOR PARKINSON’S DISEASE: YES
A.H.V. Schapira
UK
An important principle for the treatment of PD is that the introduction and use of medication must be tailored to the patient’s individual needs. Many patients may still be working when first diagnosed, their need for symptomatic therapy will depend upon the effect that the disease has on their performance at work. Other patients will need to judge the impact of symptoms on their social activities, and in all this life expectancy, quality of life and co-morbidities must be considered. The fact that the average life expectancy from diagnosis to death in PD patients is 17 years serves to emphasize the need for a long-term treatment strategy for most patients, and this should be developed and discussed with the patient at an early stage. Finally the type of drug used may be influenced by concern regarding side effects of medication, and also the cost of that medication.
The period from onset of neuronal cell dysfunction to death and then the emergence of clinical symptoms in PD is not known. In the monogenic forms of familial PD this may be over several decades. Recent studies using serial imaging in sporadic PD have suggested that the pre- symptomatic latent period from onset of dopaminergic cell loss to diagnosis is approximately six to seven years.1 Clinical progression in early PD is relatively rapid; the united Parkinson’s disease rating scale (UPDRS) deteriorates by 8-10 points in the first year and this is associated with a significant decline in quality of life.
Traditionally, treatment for PD has been withheld until symptoms have sufficient impact upon function in the workplace, social or domestic life. This view arose and developed during the levodopa era and became established teaching, 4 but perhaps deserves re-evaluation given the range of new treatments now available. We have proposed that in the appropriate patient, early symptomatic treatment is beneficial in the short and long term to improve motor control and quality of life, and can be achieved without an increase in the frequency of motor complications.5 Furthermore, we have suggested that early correction of the basal ganglia functional abnormalities caused by dopaminergic cell loss and dopamine deficiency is a means to support the intrinsic physiological compensatory mechanisms and both limit and delay the circuitry changes that evolve as PD progresses. Review of the outcomes of the DATATOP, ELLDOPA and TEMPO studies supports such a proposition.5
Once the decision has been made to initiate therapy, the specific drug chosen will depend upon the individual patient characteristics. Effective symptomatic control, good side effect profile and consideration of the need to delay motor complications all play a role in selection.6 The drugs most commonly used as first therapy are dopamine agonists, monoamine oxidase inhibitors or levodopa. The way in which these drugs are used is important. For instance, dopamine agonists are often used at too low a maintenance dose. The dose, frequency and combination of a COMT inhibitor with levodopa are important elements in enhancing the efficacy of levodopa7. The sequence and combination of these drugs may also be important in designing the best long-term strategy for any given patient.
References: 1. Hilker R, Schweitzer K, Coburger S, Ghaemi M, Weisenbach S, Jacobs AH et al. Nonlinear progression of Parkinson disease as determined by serial positron emission tomographic imaging of striatal fluorodopa F 18 activity. Arch Neurol. 2005; 62:378-82. 2. Shultz CW, Oakes D, Kibbutz K, Beal MF, Haas R, Plumb S et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002; 59:1541-50. 3. Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004; 351:2498-508. 4. Marsden CD, Parkes JD. Success and problems of long-term levodopa therapy in Parkinson's disease. Lancet. 1977; 1:345-49. 5. Schapira AH, Obeso J. Timing of treatment initiation in Parkinson's disease: A need for reappraisal? Ann Neurol. 2006; 59:559-62. 6. Schapira AH Treatment options in the modern management of Parkinson’s disease. Arch Neurol 2007; 64:1083-1088 7. Schapira, A.H., M.Emre, P.Jenner and W.Poewe. Levodopa in the treatment of Parkinson's disease. Eur.J.Neurol. (2009) in press.
NEW DRUGS FOR PARKINSON’S DISEASE: SAFINAMIDE
A.H.V. Schapira
UK
Pharmacology: Safinamide is a water soluble, orally active aminoamide derivative with multiple actions. It is a potent, highly selective and reversible inhibitor of monoamine oxidase B (MAOB) and dopamine re-uptake. It blocks voltage dependent sodium channels by preferentially interacting with the inactivated channel, modulates N-type calcium channels and reduces glutamate release.
Safinamide is rapidly absorbed after an oral dose reaching a peak plasma level in 1.8-2.0 hours and has an elimination half life in humans of 21-24 hours. In non-human primates, safinamide produces a brain to plasma concentration ratio of 9, probably reaching high micromolar levels in the central nervous system. Thus a dose of 100mg in human clinical trials might be anticipated to produce brain concentration of the order of 30uM. The drug is 92% bound in plasma and only a small proportion is excreted unchanged. There is no significant accumulation at steady state.
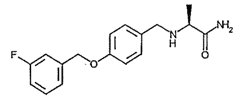 Laboratory studies: In the MPTP rodent model of PD, there is loss of dopamine terminals and a shift in metabolism of dopamine from MAOA to MAOB. In this model, safinamide increases dopamine levels by 60% when co-administered with levodopa. In the 6-hydroxydopamine model, a single dose of safinamide reversed the ‘wearing off’ response to levodopa and this effect was greater than that seen with MK801, a glutamate inhibitor. In rat primary cortical neurons safinamide protected against veratridine induced degeneration. This effect is mediated by the blockade of the voltage dependent sodium and calcium channels. The drug is also protective against kainic acid induced cell loss in hippocampal neuronal cultures.
After long-term administration to monkeys, oral safinamide significantly increased dopamine levels in putamen and prefrontal cortex, without affecting regions that are devoted to rewarding/reinforcing circuitry e.g. mesolimbic structures. Long-term administration of safinamide was not associated with pharmacological tolerance. These observations raise the possibility that safinamide may be able to improve motor function and some aspects of non-motor function in PD.
Clinical studies in early PD: In a 3 month placebo controlled randomized study, 172 patients with early PD were randomized to placebo or two doses of safinamide: 0.5 or 1.0 mg/kg. At 3 months, 37.5% (p<0.02) of patients on the high dose (mean 70mg/day) had at least a 30% improvement in UPDRS III, compared to 30.9% of the low dose and 21.4% of the placebo. This study showed that the higher dose of safinamide adjunct to dopamine agonist monotherapy produced a significant increase in UPDRS III response rate (47.1%, p<0.03) compared to placebo (20.6%); the lower dose of safinamide was not significant (36.4%). The mean improvement in motor score was 4.7 compared to baseline for the higher dose (p<0.05), 4.0 for the lower dose (ns) and 1.4 for placebo. The statistical benefit of the lower dose may have been undermined by the high placebo response. Nevertheless, the significant response in motor performance with safinamide added to a dopamine agonist (either ergot or non-ergot) is of great interest, particularly given the current use of agonists as an alternative to levodopa in early disease. Safinamide thus has the potential to extend the duration of effective PD treatment prior to levodopa introduction. It is of note that the higher dose was anticipated to produce pharmacological effects of safinamide beyond MAOB inhibition i.e. ion channel blockade and inhibition of glutamate release.
The benefit of safinamide added to dopamine agonist monotherapy was reproduced in a small open label study over 6 weeks. There was a rapid (by 2 weeks) and significant improvement in UPDRS III which reached 4.2 (p<0.001) by the end of the study.
Safinamide appears to be well tolerated. In the placebo controlled study, adverse events were less common than reported for placebo.
Clinical studies in advanced PD: A large double blind controlled study randomized 669 PD patients on stable levodopa with mean H&Y stage 2.8 and mean daily off period of 5.2 hours, to placebo or 50 or 100mg/day of safinamide over 24 weeks. The primary outcome measure was increase in daily ‘on’ time. Completion rate was 89%. On time without troublesome dyskinesias was increased more than placebo by 0.5 and 0.7 hours for the low and higher doses respectively. There was no increase in time with troublesome dyskinesias. Off time was reduced by 0.6 hours for both safinamide doses. Mean UPDRS II and III were significantly improved for both doses compared to placebo. Treatment emergent side effects included dyskinesias, both doses were generally well tolerated.
Conclusions: Current therapy for PD most commonly involves the use of levodopa, a dopamine agonist or MAOB inhibitor. The use, sequence and combination of these drugs will depend upon the individual characteristics of the patient. Levodopa may be combined with a COMT inhibitor to enhance its effectiveness and duration of action and improve wearing off; amantadine may be used to reduce dyskinesias. The properties of safinamide demonstrated to date suggest that it will be an important addition to the armamentarium of drugs for PD. It has so far shown that it can improve motor control in patients with early or advanced PD, with benefits seen in those patients already taking either a dopamine agonist or levodopa. Thus safinamide will fit neatly into current treatment algorithms for the management of both early and more advanced disease. However, further clinical development of this drug continues and the results of additional clinical trials are awaited.
References: 1. Onofrj et al Expert Opin. Investig. Drugs. 2008; 17:1-11. 2. Schapira AH. Arch Neurol 2007; 64: 1083-1088. 3. Caccia C et al. Neurology 2006; 67: S18-S23. 4. Stocchi F et al. Neurology 2004; 63: 746-748. 5. Borgohain R et al. Parkinsonism Relat Disord 2007; 13: S99
Conflicts of Interest
Professor Schapira has served as an advisor to GSK, Orion- Novartis, BI, Teva-Lundbeck and EMD Serono.
IV TPA BEYOND 3H: IS IT SUITABLE FOR EVERYONE?
P. Schellinger
Medical Faculty, Universität Erlangen, Germany
rt-PA currently is approved after CT-based exclusion of intracranial haemorrhage in a 3h time window after stroke symptom onset. Inclusion criteria are different in Europe and America, the former being somewhat stricter regarding stroke severity, patient age and blood pressure management, albeit the recommendations for thrombolysis of the European and American Stroke Societies are rather congruent especially regarding risk factors for bleeding complications such as high blood pressures and blood glucose as well as imaging findings on non contrast CT.
With the publication of ECASS 3 last year, the time window has been opened up to 4.5 hours. ECASS 3 was a RCT fulfilling the highest trial standards in keeping with the times. The inclusion and exclusion criteria were identical to the European SOP except of the time window between 3 and 4.5 hours. Approval will very likely change in Europe, this has already been filed with the EMEA.
Is rt-PA suitable for everyone beyond 3h? For the sake of discussion I will not make my arguments in this abstract, but the answer is like always: Yes and No!!
COAXIA
P. Schellinger
Medical Faculty, Universität Erlangen, Germany
Hemodynamic augmentation is a technique applicable to all low- flow situations in cerebral disease. Situations that come to mind especially are vasospasm and acute ischemic stroke. The coaxia NeuroFlo device introduces via a 7 French sheath a double inflatable balloon catheter. These balloons are inflated first proximal then distal to the renal artery up to a partial aortic occlusion of approximately 70 percent and maintaining renal artery flow. Thereby a cerebral blood flow increase of 30% on average is induced. This has been verified in animal studies but also by several imaging modalities such as PET, MRI, CT and angiography in humans.
Currently the clinical study program with the NeuroFlo catheter includes several series about the use in vasospasm after SAH, which is not the current topic. In ischemic stroke 2 pilot trials have been completed using the NEuroFLo technique in ischemic strokes up to 24h based on a PI/DWI mismatch (Flo24) and in a preliminary series of more than 20 patients as an adjunct to thrombolysis with rt-PA. The presumed effect of NEuroFlo is a stabilization of collaterals and potentially an augmentation of autolytical or rt-PA mediated recanalization.
The pivotal study has by now recruited more than 400 patients of nearly 500 planned.
IS AED DISCONTINUATION IN SEIZURE-FREE PATIENTS DANGEROUS? THE ANSWER IS YES
D. Schmidt
Epilepsy Research Group Berlin, Germany
Notwithstanding its potential benefits, discontinuation of AEDs in seizure-free patients may be dangerous for two reasons. First, seizure recurrence is seen in as many as 50-70% of high-risk patients. High-risk patients are those who tried unsuccessfully to stop AEDs in the past, patients with juvenile myoclonic epilepsy, and those with symptomatic focal or generalized epilepsy, and adults. Even in so-called low-risk patients, the risk of seizure recurrence is 20-30%. The second major risk is that, at least in some, but not all studies, reinstitution of AEDs does not guarantee immediate and full seizure remission in many patients. It may take years for some patients to become seizure-free again after restarting AEDs, if ever. A third reason why to consider the dangerous consequences of stopping AEDs is simply that the physician may violate the principle of “primum non nocere”. Any physician who propagates stopping AEDs is in a difficult position in case of seizure recurrence. Although being kept on AEDs is unfortunately no guarantee to remain seizure-free in the long run, patients and their lawyers intuitively tend to attribute the seizure recurrence to the discontinuation of the treatment. It has been shown that AED discontinuation does increase the risk of seizures in the 1- to 2-year period after discontinuation. As a consequence, seizure recurrence may erode confidence in the physician- patient relationship. Seizure recurrence in a patient who was seizure free for many years can have dramatic medical, social and psychological consequences for the patient. These include loss of driver's license, employment, injury and loss of self-esteem. Finally, the reason why discontinuation may have dangerous consequences is that it unintentionally deprives patients from treatment who continue to need AEDs for seizure protection. Although it is controversial if discontinuation of AEDs modifies the long-term prognosis of a person's epilepsy, it is clear that it does increase the risk of seizures in the 1- to 2- year period after discontinuation. The serious and substantial short-term risks weigh against discontinuation of AEDs in seizure-free patients with partial seizures, and neurological handicap.
LACOSAMIDE
D. Schmidt
Epilepsy Research Group Berlin, Germany
Lacosamide (Vimpat; UCB), (R)-2-acetamido-N-benzyl-3-meth- oxypropionamide, is a new antiepileptic drug for adjunctive treatment of refractory focal seizures in adults. In vitro studies have identified two properties of lacosamide that might be relevant to its therapeutic effects. First, lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Second, lacosamide binds to collapsin response mediator protein 2 (CRMP2), which is involved in neuronal differentiation, control of axonal outgrowth and probably also epileptogenesis. Its pharmacokinetic characteristics have been explored in adults with epilepsy or diabetic neuropathic pain. After oral administration, lacosamide is rapidly and completely absorbed. An elimination half-life of 13 hours allows for twice-daily dosing. Lacosamide has a low potential for drug-drug interactions. Lacosamide (at doses of 200 mg, 400 mg or 600 mg per day) was studied in three randomized, placebo-controlled clinical trials with a 12-week maintenance period in a total of 1,308 adults with chronic, uncontrolled partial-onset seizures with or without secondary generalization. In placebo-controlled clinical trials, lacosamide has demonstrated efficacy as adjunctive therapy for reduction of seizure frequency in adult patients with uncontrolled focal-onset seizures, and has been generally well tolerated. Overall in the three trials, the proportion of patients with a 50% reduction in seizure frequency was 23%, 34% and 40% for placebo, lacosamide 200 mg per day and lacosamide 400 mg per day, respectively. A statistically significant reduction in 28-day seizure frequency (baseline to maintenance phase)
As compared with the placebo group was observed with lacosamide 200 mg per day in the second trial, 400 mg per day in all three studies and 600 mg per day in both studies with this dose. The efficacy of lacosamide
600 mg per day was similar to that of lacosamide 400 mg per day, but patients were less likely to tolerate this dose because of CNS- and gastrointestinal-related adverse effects including dizziness, headache, nausea and diplopia. The maximum recommended dose is 400 mg per day. When used as short-term replacement for oral lacosamide, intravenous lacosamide has a comparable safety profile to oral lacosamide. Results from clinical trials to date suggest that lacosamide may be a useful pharmacological treatment option for patients with refractory focal-onset seizures in adults.
PATIENTS WITH MEDICATION OVERUSE HEADACHE SHOULD NOT UNDERGO WITHDRAWAL PRIOR TO INITIATING PREVENTATIVE MEDICATION
J. Schoenen
Headache Research Unit, Department of Neurology & GIGA-Neurosciences, University of Liege, Liege, Belgium
Medication overuse headache (MOH) (ICHD-II 8.2) (Headache Classification Committee 2006) evolves insidiously from episodic migraine or tension-type headache because of overconsumption of analgesics, ergotamine or triptans. It affects 1–2% of the general population (Scher et al. 2003) including adolescents (Wang et al. 2006), but 15-20% of patients attending specialized headache centers. The critical duration of drug overuse before MOH develops and the critical number of monthly doses required vary between individuals and are lower for triptans (1.7 years and 19 doses respectively) than for analgesics (4,8 years and 114 doses) ; they are intermediate for ergotamine preparations (Limmroth et al. 2002). By contrast, withdrawal headaches last longer after analgesics or ergotamine overuse (up to 14 days) than after triptan overconsumption (5-6 days) (Katsarava et al. 2001). This suggest that MOH occurs more rapidly and for an average lower intake frequency in triptan users, but in these patients MOH is also more easily manageable than in ergotamine or analgesic users.
The precise neurobiological mechanisms underlying this complication of episodic headaches remain elusive. The causative role played by drug overuse is still controversial and not well understood. The fact that most patients improve after weaning suggests nonetheless that the excessive drug intake is a major chronifying and aggravating factor in most CDH patients (Schoenen et al. 1989; Diener 1993, Scher et al. 2003; Zwart et al 2003). It is a common observation that daily analgesic consumption does not cause headache in patients without primary headache, but it does so in migraineurs in whom these drugs were prescribed for osteoarthritis pain (Bahra et al. 2003).
Abnormalities of central pain processing (Fusco et al. 1997) and central neurotransmitters (Schoenen et al., 1987; Srikiatkhachorn et al. 1994; Nicolodi et al. 1997; Anselmi et al. 1997 et al.; Hering et al. 1993) have been reported and psychopathological features have been pinpointed in these patients (Verri et al. 1998; Curioso et al. 1999). More than 60% of MOH patients fulfill DSM-IV criteria for addictive behavior (Radat et al 2008) and they have a higher prevalence of substance dependence in their personal and family history (Radat & Swendsen 2005). In a recent FDG-PET study of 16 migraineurs with MOH before and after analgesics withdrawal we found a persistent hypometabolism of the medial orbitofrontal cortex, comparable to the one described after withdrawal in substance abuse (Fumal et al. 2005). The orbitofrontal cortex plays a pivotal role in drive, decision-making and drug dependence. We postulate that its hypoactivity predisposes certain migraineurs to MOH and to relapse after withdrawal.
There is no unique management strategy for MOH patients. Medication withdrawal is considered to be a prerequisite for the effectiveness of preventive treatments and headache improvement, but this may be only partly true. In a study of the effect of topiramate in chronic migraineurs, there was an improvement even in those who were overusing acute medications. However, this was a modest effect at the limits of clinical usefulness: 23% decrease in monthly migraine days (Diner et al 2007). In another most interesting 1-year open-labeled study with intention-to-threat analysis (Hagen et al 2009), no significant difference in outcome was found at the end of the follow-up period between patients who received a prophylactic treatment from the start on without previous detoxification (53% with ≥ 50% reduction in monthly headache days) and those who were detoxified before starting preventive therapy (25%). The fact that many preventive anti-migraine drugs become effective only after several weeks of treatment is another good reason for not delaying their prescription. There is at present no proof that on the long term in-patient detoxification combined or not with a subsequent in-patient rehabilitation program is more efficient and cost-effective than out-patient management. In the few comparative studies performed up to now, there were no significant differences in short term outcome between different management strategies (Rossi et al 2006).
We have therefore adopted since many years the following management program in our center:
- Abrupt withdrawal of analgesics/ergotamine;
- NSAIDs (ibuprofen, naproxen or indomethacine) (max.2- 3d/week) or triptans (max.1d/week) to control headache;
- Acamprosate p.o. (333mg t.i.d.), a glutamate antagonist and GABA agonist also used in alcoholism, for 2 weeks to control withdrawal symptoms;
- Immediate prophylactic therapy in most patients valproate or topiramate, in others methysergide, beta-blockers and riboflavin;
- Psychological support is necessary, including from caregivers
- Patients are treated as outpatients, followed by their GP and reconsult after 3 to 4 months;
- because of failure to withdraw from drug overuse, intolerance to treatment, or lack of support by caregivers, ± 1 % patients of patients are hospitalized for 7 days. They receive i.v. infusions of clomipramide and tiapride, in addition to oral prophylactics;
- The i.v. dihydroergotamine program which is popular in Northern America is seldom used; however, i.m. DHE associated with tiapride is given to some outpatients;
- in a retrospective survey, the success rate of this program was 90 % at 1 month, 60% at 12 months and 50% at 10 months (Schoenen et al. 1989).
References: Anselmi B et al. Serum beta-endorphin increase after intravenous histamine treatment of chronic daily headache. Recenti Prog Med 1997; 88: 321-324: Bahra A, Walsh M, Menon S, Goadsby PJ. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache 2003; 43: 170-190; Curioso EP, Young WB, Shechter AL, Kaiser RS. Psychiatric comorbidity predicts outcome in chronic daily headache patients. Neurology 1999; 52, Suppl 2: S67.004.
Diener HC. A personal review of the classification and definition of drug dependence headache. Cephalalgia 1993; 13, Suppl 12 : 68-71; Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ; TOPMAT-MIG-201(TOP-CHROME) Study Group. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia. 2007;27(7):814-23; Fumal A, Laureys S, Di Clemente L, Boly M, Bohotin V, Vandenheede M, Coppola G, Salmon E, Kupers R, Schoenen J. Orbitofrontal cortex involvement in chronic analgesic overuse headache evolving from episodic migraine. Brain 2006; 129:543-50; Fusco BM, Colantoni O, Giacovazzo M. Alteration of central excitation circuits in chronic headache and analgesic misuse. Headache 1997; 37 : 486-491; Hagen K, Albretsen C, Vilming ST, Salvesen R, Grønning M, Helde G, Gravdahl G, Zwart JA, Stovner LJ. Management of medication overuse headache: 1-year randomized multicentre open-label trial. Cephalalgia. 2009;29(2):221-32; Headache Classification Committee: J Olesen, M-G Bousser, H-C Diener, D Dodick, M First, PJ Goadsby, H Göbel, MJA Lainez, JW Lance, RB Lipton, G Nappi, F Sakai, J Schoenen, SD Silberstein & TJ Steiner. Cephalalgia 2006; 26: 742-746; Hering et al. Cellular adaptation in migraineurs with chronic daily headache. Cephalalgia 1993; 13 : 261- 266; Katsarava Z, Fritsche G, Muessig M, Diener HC, Limmroth V. Clinical features of withdrawal headache following overuse of triptans and other headache drugs. Neurology, 2001, 57, 1694-8; Limmroth V, Katsarava Z, Fritsche G, Przywara S, Diener HC. Features of medication overuse headache following overuse of different acute headache drugs. Neurology, 2002, 59, 1011–1014; Nicolodi et al. Modulation of excitatory amino acid pathways: a possible therapeutic approach to chronic daily headache associated with analgesic drugs abuse. Int J Clin Pharmacol Res 1997; 17 (2-3):97-100. Radat F, Swendsen J. Psychiatric comorbidity in migraine: a review. Cephalalgia, 2005, 25, 165–178; Radat F, Creac'h C, Guegan-Massardier E, Mick G, Guy N, Fabre N, Giraud P, Nachit-Ouinekh F, Lantéri-Minet M. Behavioral dependence in patients with medication overuse headache: a cross-sectional study in consulting patients using the DSM-IV criteria. Headache. 2008;48 (7):1026-36; Rossi P, Di Lorenzo C, Faroni J, Cesarino F, Nappi G. Advice alone vs. structured detoxification programs for medication overuse headache: a prospective, randomized, open-label trial in transformed migraine patients with low medical needs. Cephalalgia. 2006; 26(9):1097-105; Scher A.I., Stewart W.F., Ricci J.A... Lipton R.B. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003; 106: 81–89; Schoenen J, Malais P, Dresse A. Urinary excretion of 3-methoxy-4-hydroxy-phenylglycol (MHPG) in headache patients : clinical relations. Cephalalgia 1987; 7, Suppl. 6: 29-31; Schoenen J, Lenarduzzi P, Sianard-Gainko J Chronic headaches associated with analgesics and/or ergotamine abuse : a clinical survey of 434 consecutive out-patients. In "New Advances in Headache Research". Clifford Rose F., ed. Smith-Gordon, 1989: 255-259; Srikiatkhachorn A et al. Up-regulation of 5-HT2 receptor: a possible mechanism of transformed migraine. Headache 1994; 34 (1) : 8-11; Verri AP, Proietti Cecchini A, Galli C, Granella F, Sandrini G, Nappi G. Psychiatric comorbidity in chronic daily headache. Cephalalgia 1998; 18 Suppl 21 : 45-49; Wang Shuu-Jiun, Fuh Jong-Ling, , Lu Shiang-Ru, Juang Kai-Dih. Chronic daily headache in adolescents. Prevalence, impact, and medication overuse. Neurology 2006; 66:193-197; Zwart JA, Dyb G, Hagen K, Svebak S, Holmen J. Analgesic use: a predictor of chronic pain and medication overuse headache: the Head-HUNT Study. Neurology, 2003, 61, 160-164.
THE ORIGIN OF MIGRAINE ATTACKS: CORTICAL SPREADING DEPRESSION IS NOT THE FIRST PHASE OF EACH MIGRAINE ATTACK
J. Schoenen
Headache Research Unit, Department of Neurology & GIGA-Neurosciences, University of Liege, Liege, Belgium
There is strong evidence from clinical (Milner 1958) and functional brain imaging (Lauritzen & Olesen 1984; Cao et al 1999; Hadjikhani et al 2001) studies that the migrainous aura is due to cortical spreading depression (CSD). CSD is able in animals to activate the trigeminovascular system which is the neuronal pain-signaling system of the brain thought to be responsible for the migraine headache (Moskowitz et al 1993). It has been underscored, however, that in migraine with aura there is no obligatory chronological relation between aura and headache, as the former may supervene without the latter or coincide with the headache in a substantial number of patients (Goadsby 2001).
Ayata et al (2006) have found in rat that all preventive anti- migraine drug they tested, i.e. topiramate, valproate, DL-propranolol, amitriptyline and methysergide, inhibited CSD occurrence and threshold after chronic administration of at least 4 weeks. Hence they suggested that suppression of CSD may be the common mechanism for the effect of preventive anti-migraine drugs not only in migraine with aura, but also in migraine without aura where CSDs would occur in brain areas that are clinically silent.
These hypotheses cannot be taken without reservation for several reasons related to the pathophysiology of migraine without aura and the pharmacological profile of drugs effective in migraine prevention (Schoenen 2006, Wolthausen et al 2008)..
Besides the fact that it seems unlikely that CSD would remain restricted to clinically silent areas for the whole lifespan, there is no convincing evidence for a CSD-like event on imaging studies in migraine without aura patients (Weiller et al 1995, Olesen et al 1982). In a recent PET study a modest posterior hypoperfusion was found during attacks of migraine without aura, but the recordings were made >3 hours after the beginning of the attack and the results have not been reproduced yet (Denuelle et al 2008). Moreover, CSD does not produce CGRP release which is thought to be characteristic of migraine headaches (Ebersberger et al. 2001; Bolay et al. 2002).
The genetic abnormalities found in familial hemiplegic migraine (Ophoff et al 1996) promote CSD in knock-in mice (Van den Maagdenberg et al 2004), but they have not been found in the common forms of migraine with or without aura after already more than 10 years of intensive studies.
Although the relevant mechanism of action of preventive anti- migraine drugs remains elusive, several pharmacological and clinical data suggest that a single common mechanism of action is unlikely. On the one hand, several compounds which are able to suppress CSD in the animal model like carbamazepine (Chen et al 2005) and clonidine (Richter et al., 2005), have no effect in migraine (Fumal & Schoenen 2008, Evers et al., 2009). On the other hand, lamotrigine which was not assessed in Ayata et al’s study is a drug of major interest. It induces in the human brain distinct neurophysiological changes compared to topiramate (Smith et al. 2006). More importantly, based on clinical trials and experience, it is effective in migraine with (D’Andrea et al.1999; Pascual et al 2004; Lampl et al. 2005; Fumal & Schoenen 2006) but not in migraine without aura (Steiner et al. 1997; Pascual et al 2004). Lamotrigine might thus be expected to have a potent suppressing action on CSD. By contrast, riboflavin, a nutriceutical enhancing mitochondrial energy mechanism, would not be expected to have an effect on CSD and concordantly does not modify cortical excitability as assessed by evoked potentials (Sándor et al. 2000). Nonetheless, it has been proven effective at high doses in migraine prophylaxis in randomized controlled trials (Schoenen et al. 1998, Boehnke et al. 2004). We have indeed recently found that in the KCl-induced CSD rat model used in Ayata et al’s study chronic administration of lamotrigine is the most potent inhibitor of CSD, while valproate has only a mild effect on CSD propagation and riboflavin no beneficial effect (Bogdanov et al 2009). Interestingly, another compound, tonabersat, which inhibits connexins, may have a similar profile. It strongly inhibits CSD (Smith et al 2000) and preliminary clinical trials suggest that it might be effective in migraine with aura (Hague et al 2009), but not in migraine without aura (Goadsby et al 2009).
There is thus at present no proof that CSD inhibition is a “sine qua non” condition for therapeutic efficacy in migraine with, and especially without aura. As a corollary it seems not necessary to postulate that all migraine types start with CSD; this is probably the case only for migraine with aura. All preventive anti-migraine drugs have various pharmacological properties which may explain their clinical efficacy (Fumal & Schoenen 2008).
References: Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhaupl KM and Arnold G. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol 2004, 11 (7): 475-477; Bogdanov V., Chauvel V., Multon S., Makarchuk M., Schoenen J. Preventive anti-migraine drugs differentially affect KCl-induced cortical spreading depression in rat. Cephalalgia 2009; 29: 131; Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002; 8(2):136-42; Cao Y, Welch KM, Aurora S, Vikingstad EM. Functional MRI-BOLD of visually triggered headache in patients with migraine. Arch Neurol. 1999; 56 (5):548-54; Chen G, Gao W, Reinert KC, Popa LS, Hendrix CM, Ross ME, Ebner TJ. Involvement of kv1 potassium channels in spreading acidification and depression in the cerebellar cortex. J Neurophysiol. 2005;94(2):1287-98; D’Andrea G, Granella F, Cadaldini M, et al. Effectiveness of lamotrigine in the prophylaxis of migraine with aura: an open pilot study. Cephalalgia 1999;19:64–6; Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Posterior cerebral hypoperfusion in migraine without aura. Cephalalgia 2008; 28(8):856-62; Ebersberger A, Schaible HG, Averbeck B, Richter F. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache? Ann Neurol. 2001;49(1):7-13; Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS. EFNS guideline on the drug treatment of migraine--revised report of an EFNS task force. Eur J Neurol. 2009; 16(9):968-81; Fumal A., Schoenen J. Effectiveness of lamotrigine in the prophylaxis of migraine with aura: an open study. Cephalalgia 2006, 11: 1369; Fumal A. and Schoenen J. Current migraine management – patient acceptability and future approaches. Journal of Neuropsychiatric Disease and Treatment 2008; 4:1043-57; Goadsby PJ. Migraine, aura, and cortical spreading depression: why are we still talking about it? Ann Neurol. 2001;49(1):4 -6; Goadsby PJ, Ferrari MD, Csanyi A, Olesen J, Mills JG, for the Tonabersat TON-01-05 Study Group. Randomized double-blind, placebo -controlled proof-of-concept study of the cortical spreading depression inhibiting agent tonabersat in migraine prophylaxis. Cephalalgia 2009; 29: 742–50; Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, Kwong KK, Cutrer FM, Rosen BR, Tootell RB, Sorensen AG, Moskowitz MA.Mechanisms of migraine aura revealed by functional MRI in human cortex. Proc Natl Acad Sci USA 2001;98:4687– 4692; Hague AW, Asghar MS, Schytz HW, Christensen K, Olesen J. Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. Lancet Neurol 2009. published online June 30; Lampl C, Z Katsarava, H-C Diener and V Limmroth. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. J. Neurol. Neurosurg. Psychiatry 2005; 76; 1730-1732
Lauritzen M, Olesen J. Regional cerebral blood flow during migraine attacks by Xenon-133 inhalation and emission tomography. Brain 1984; 107: 447-61; Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci 1993;13:1167–1177; Olesen J, Lauritzen M, Tfelt-Hansen P, Henriksen L, Larsen B. Spreading cerebral oligemia in classical- and normal cerebral blood flow in common migraine. Headache 1982; 22(6): 242-8; Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996;87:543-552; Pascual J, Caminero AB, Mateos V, Roig C, Leira R, García-Moncó C, Laínez MJ. Preventing disturbing migraine aura with lamotrigine: an open study. Headache. 2004; 44(10):1024-8; Richter F, Mikulik O, Ebersberger A, Schaible HG. Noradrenergic agonists and antagonists influence migration of cortical spreading depression in rat-a possible mechanism of migraine prophylaxis and prevention of postischemic neuronal damage. J Cereb Blood Flow Metab. 2005;25(9):1225-35; Sándor P.S., Áfra J., Ambrosini A., Schoenen J. Prophylactic treatment of migraine with beta-blockers and riboflavin : differential effects on the intensity dependence of auditory evoked cortical potentials. .Headache 2000, 40: 30-35; Schoenen J., Jacquy J., Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis: a randomized controlled trial. Neurology 1998, 50: 466-470; Schoenen J. Future Preventive therapy: are there promising drug targets? Headache Currents 2006, 3: 108-111
Smith ME., Gevins A., McEvoy LK., Meador KJ., Ray PG., Gilliam F. Distinct Cognitive Neurophysiologic Profiles for Lamotrigine and Topiramate. Epilepsia, 47(4):695–703, 2006; Smith MI, Read SJ, Chan WN, et al. Repetitive cortical spreading depression in a gyrencephalic feline brain: inhibition by the novel benzoylaminobenzopyran SB-220453. Cephalalgia 2000; 20: 546–53.
Steiner TJ, Findley LJ, Yuen AW. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia 1997;17:109–12; van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, van de Ven RC, Tottene A, van der Kaa J, Plomp JJ, Frants RR, Ferrari MD. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41(5):701-10; Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995; 1: 658-660.
Wolthausen J, Sternberg S, Gerloff C, May A. Are cortical spreading depression and headache in migraine causally linked? Cephalalgia 2009; 29(2):244-9.
TRIPTANS MUST BE GIVEN EARLY WHEN THE HEADACHE IS MILD TO BE EFFECTIVE IN MIGRAINE: MYTH OR REALITY?
J. Schoenen
Headache Research Unit, Department of Neurology & GIGA-Neurosciences, University of Liege, Liege, Belgium
There is clinical and scientific evidence that triptans are more effective in migraine if they are given early in the attack when pain is mild. Pain-free response rates in particular are clearly higher for early triptan treatment. This is irrefutable, but it is the case for any anti-migraine treatment including NSAIDs and thus not specific for triptans. It has been stated that triptans are (or not?) effective when the headache is more severe because of central sensitization reflected clinically by cutaneous allodynia. We have shown in a study where patients auto-evaluated dynamic mechanical allodynia that treatment efficacy, be it for a triptan or a combination of a triptan and an NSAID is not chiefly drive by allodynia but by pain intensity at the time of treatment (Schoenen et al 2008).
There are several reasons why the general rule should not be to recommend to patients to take a triptan when their headache is mild. First, several studies have shown that simple NSAIDs are as effective as triptans for the treatment of mild and moderate attacks. Second, in most patients the severity varies between attacks and some patients recognize from the beginning on which attack will stay moderate and which will become severe. Third, there is evidence that in patients with frequent attacks the use of triptans favors occurrence of medication overuse headache while NSAIDs rather have a protective effect.
Taken together, these data suggest that patients should be recommended to start their attack treatment with an NSAID at a sufficient dose at a stage where the headache is mild and their impression is that the attack will not be rapidly excruciating. A triptan should be taken if the attack is not interrupted after 1-2h. The patient must be given the pharmacological armamentarium to apply this “step-wise within attack” approach. Clinical practice and formal studies do not support the general assertion that triptans are ineffective when given at a stage when the headache is severe. Injectable sumatriptan in particular remains effective at any time during the attack
References: Schoenen J., De Klippel N., Giurgea S., Herroelen L., Jacquy J., Louis P., Monseu G., Vandenheede M. Almotriptan and its combination with aceclofenac for migraine attacks: a study of efficacy and the influence of auto-evaluated brush allodynia. Cephalalgia 2008, 28:1095-1105.
QUANTUM MECHANICS AND SELF-DIRECTED NEUROPLASTICITY
J. Schwartz
UCLA Department of Psychiatry Los Angeles, CA USA
Neurobiological research generally assumes that brain mechanisms alone will ultimately suffice to explain all psychologically described phenomena. This assumption stems from the idea that all causal mechanisms relevant to neuroscience can be formulated solely in terms of the principles of classic Newtonian physics. Thus, terms having intrinsic experiential content (e.g. ‘feeling’, ‘observing’ and ‘effort’) are not included as primary causal factors. This theoretical perspective is dictated by ideas about the natural world that have been known to be fundamentally incorrect for more than three-quarters of a century. Contemporary physical theory differs profoundly from classic Newtonian physics on the important matter of how the consciousness of human agents enters into the causal dynamics of empirical phenomena. The new quantum principles contradict the older idea that mechanical processes alone can account for all observed empirical data. Contemporary quantum physical theory brings directly and irreducibly into the overall causal structure certain psychologically described choices made by human agents about how they will act. This key development is applicable to neuroscience, and it provides neuroscientists and psychologists with an alternative conceptual framework for describing neural processes. The new framework and specifically the well described physical principle known as quantum Zeno effect, enable scientists and clinicians to better understand the neuroplastic mechanisms relevant to the growing number of studies demonstrating the capacity of directed attention and mental effort to systematically alter brain function. Clinical and neuropsychological findings from research on obsessive-compulsive disorder, placebo effect, stroke, and normal human psychology will be discussed and elucidated in light on this theoretical paradigm shift.
WHAT IS THE BEST TREATMENT FOR ADVANCED FLUCTUATING PD: DBS?
E. Seigneuret
France
Deep brain stimulation (DBS) has proved to be an effective surgical treatment for advanced Parkinson's disease (PD) with motor complications, with significant advantages in morbidity-mortality and quality of life. Currently of course, DBS means chronic stimulation of the Sub Thalamus Nucleus (STN).
But is it the best treatment for advanced fluctuating PD?
In our experience, the most important eligibility criteria for DBS are: a correct diagnosis of idiopathic PD, severity of illness, a consistent levodopa response and absence of cognitive impairment.
Fluctuating motor complication, improve dramatically after DBS, because the continuous stimulation, and decrease of medical treatment.
Some studies proved also that non-motor fluctuating complications are improved: pain fluctuations, dysautonomic fluctuations, cognitive fluctuations. This phenomenon may be explained by the stabilization of synaptic dopamine concentrations in the striatum and may be attributed to the alleviation of levodopa-related fluctuations.
Some symptoms can be worsened or at least none ameliorated by STN DBS: freezing, verbal fluency and dysarthria. Even, sometimes every symptom is not equally improved. In these cases, dual target can be used: STN-VIM, STN-GPI, and more recently new target as Pediculo-pontine nucleus with low-frequency electrical stimulation (25 Hz) for axial symptoms.
However, the long-term stimulation is possible without alleviation of the therapeutic effect (18 years for the first Grenoble patient).
Therefore, DBS is a good long-term treatment for advanced fluctuating PD, especially since the multiple and new targets.
NEW PLAYERS IN MS: HAS THE TIME COME TO SWITCH TO ORAL DRUGS AS FIRST-LINE THERAPY IN MS?
WHERE TO GO IN A PATIENT WITH HIGHLY ACTIVE MS?
A. Siva
Department of Neurology, Cerrahpasa School of Medicine, Istanbul University, Istanbul, Turkey
Within the next few years we will have a number of new drugs for the long term treatment of multiple sclerosis (MS), some as oral agents (such as Cladribine, Laquinimod, Fumarate and Fingolimod) and some as monoclonal antibodies (Alemtuzumab, Rituximab). Some of these drugs are new, but others are already in the market and they are used in the treatment of some other diseases and quite likely to be approved for use in MS too.
Currently the conventional first line MS therapies, which are the interferon beta 1 group drugs and glatiramer acetate, all are used either intramuscularly or subcutaneously, in a frequency ranging from once weekly to three times weekly or every other day to everyday injections. These drugs are only partially effective and may have both some physical side effects and a psychological impact on their users. However, there is much data on their long term use and they have a very good long term safety record.
The probability of having oral drugs as an alternative to injectable long-term treatments in MS are very intriguing for patients and many, who are tired of giving frequent injections to their self are awaiting their approval by healthcare authorities, in order to switch to them. However, it is us, “the neurologists”, who will have to decide what to do, once the new drugs will be available on the market. The two major issues that we will have to address will be efficacy and safety. Preliminary data shows that these drugs are effective, with some probably having even a higher efficacy than the current treatments. But yet, it’s very early to decide whether they have a better safety record. The probability of an increased risk of infections which may have severe consequences and malignancies need to be clarified. Therefore, in MS patients who are already on interferon beta 1 group drugs or glatiramer acetate, who respond well and tolerate them, keeping the patient on their original drug may be a better option, until we will end up with more solid data concerning the new players. One other difficulty area with the new players will be what to do in treatment naïve patients?
Natalizumab and mitoxantrone are currently other approved drugs for more severe forms of relapsing or transitional MS, but due to their potential severe side effects their use are limited. Finally monoclonal antibodies such as Alemtuzumab and Rituximab are the other new players, who may be new alternatives in the more severe- highly active forms of MS. However, the increased risk of opportunistic infections with considerable morbidity and mortality, increased risk of (mostly hematologic-) malignancies, and increased autoimmunity as side effects with these drugs also limit their use as it is the case with the old boys!
In conclusion, although that in the near future we will have a number of “patient-friendly” new drugs to use in MS, treatment decisions on when to start, which one to give, in whom to switch from the old players to the new ones, how long to continue them and combination options, currently all seem to be difficult questions to answer.
THE ORIGIN OF MIGRAINE ATTACKS: MIGRAINE PAIN ORIGINATES IN THE EXTERNAL CAROTID ARTERY
E. Shevel
South Africa
Migraine is a complex condition, the pathophysiology of which remains uncertain. Although there is agreement among headache researchers on certain aspects such as the presence of trigeminovascular activation, central sensitization, heightened pain sensation, and sterile neurogenic inflammation, the significance of vasodilatation remains the subject of a great deal of controversy and confusion amongst headache experts. The objective of this presentation is to clarify the role of vasodilatation in migraine, according to the proven scientific evidence in the headache literature. The respective roles of both the intracranial and the extracranial vessels are reviewed and the evidence presented. The conclusion, based on the available hard scientific evidence, is that dilatation of the intracranial vessels does not cause migraine pain. There is compelling evidence however, that vasodilatation of the extracranial terminal branches of the external carotid artery is a cause of migraine pain.
A PLASMA BASED SIGNATURE SUITABLE FOR EARLY AD SCREENING?
H. Soares
Pfizer Global Research and Development, New London, Connecticut, USA
Alzheimer’s disease (AD) is a debilitating neurodegenerative disorder estimated to affect 5 million people in the USA alone, with incidence expected to increase four-fold over the next decade. Current NINCDS-ADRDA and DSM-IV approaches for the diagnosis of AD rely upon prolonged observation periods during which time the patient may progress through disease with associated neuropathological sequalae. Many studies utilizing CSF and imaging biomarkers have suggested that early pre-dementia stages of Alzheimer’s may, in fact, be detectable. However, CSF and imaging approaches are considered invasive and expensive. One strategy towards early diagnosis includes a simple cognitive memory test that would then be used to determine which patient would be eligible for more confirmatory CSF or imaging screening. Additional simple non-invasive screening strategies have been proposed including EEG, eye and blood tests. Recent studies have suggest an 18 multiplexed plasma protein panel can identify patients with prodromal AD suggesting multiplex panels may have potential as screening tools for early AD. However, efforts to reproduce these initial findings in a 50 patient small natural history study and in 1200 patient population study have been largely unsuccessful. The current study examines performance of a novel 189 quantitative multiplexed luminex based protein panel in differentiating AD from controls in over 300 AD and 300 matched controls. Analysis of the dataset with a combination of machine learning language and statistical approaches suggest panels containing between 7- 20 analytes can differentiate AD from controls with sensitivity and specificity of 80% respectively suggesting some utility as a screening tool for AD. Additional studies are planned to examine performance in patients with a memory complaint who then progress to dementia of the Alzheimer’s type.
SHOULD SURGERY BE OFFERED TO PATIENTS WITH EXTRA-TEMPORAL EPILEPSY IF THE MRI DOES NOT SHOW A STRUCTURAL LESION? NO
M. Sperling
USA
Surgery effectively abolishes seizures in many patients with medically refractory epilepsy. The goal of surgery is to resect an epileptogenic structural lesion, and surgery works best when histologically abnormal tissue is excised. Patients who have temporal lobe seizures fare best with surgery, with mesial temporal sclerosis serving as the most common pathological substrate. Patients with extratemporal epilepsy have lower success rates, and defining the region to be resected is often quite difficult. When a structural lesion is present, a clear target presents itself to the surgeon. However, when the MRI is normal, the epileptogenic zone must be defined by the clinical features and EEG, and precise location is often difficult to achieve. Absent a structural lesion, intracranial EEG is nearly always required a procedure which entails some morbidity and discomfort. Success rates for frontal and parietal lobe surgery may average 25-30% with lower success rates when a structural lesion is not present. The attendant risk, if eloquent cortex is near, is significant. Therefore, one may argue that surgery should generally be avoided if a clear-cut lesion is not present in the MRI, since success rates are relatively low and these operations often pose significant risk.
CONFIRMING THE DIAGNOSIS OF EARLY AD/MCI WITH BIOMARKERS IS CLINICALLY USEFUL
L. Spiru
Ana Aslan International Academy of Aging, Bucharest, Romania
At present, Alzheimer Dementia (AD) accounts for about half of all dementia cases. Even if several factors may counteract the prediction of the so called Alzheimer's Crisis (J. Arehart-Treichel, 2008), the study led in 2003 by D. Evans at the Rush-Presbyterian-St. Luke's Medical Center in Chicago demonstrated the impact of age as the greatest known risk factor for Alzheimer's. One of the most suitable responses to the looming Alzheimer's Crisis is early diagnosis – the detection of the underlying disease processes previously to the appearance of clinical symptoms, when the medical and non-medical interventions may have beneficial impact on disease’s progression. The only way to definitely diagnose Alzheimer's is through a brain autopsy, so that the struggle is actually centered on the elaboration of highly sensitive tools for early detection and differential diagnosis. Efforts are under way to identify neuroimaging and chemical biomarkers able to detect the onset of the neurodegenerative processes that culminate in clinically manifest AD dementia and which of the MCI (Mild Cognitive Impairment) subjects will subsequently progress to Alzheimer’s disease (AD). Early diagnosis is a cornerstone of preventive approaches to AD.
The presentation goes around the debate on the usefulness of biomarkers in confirming the early stage of AD – the Mild Cognitive Impairment – MCI, arguing the answer YES to this actually questioned usefulness. The short review of the MCI notion underlines the problems related to its phenotypical heterogeneity, its heuristic and methodological attempt, with focus on the need of highly accurate diagnosis tools as the first, crucial step for the prediction of the further evolution of the disease and its medical and non-medical management. The literature on biomarkers of MCI is still under development and the presentation tries a disambiguation of biomarker notion and a useful classification, insisting on the main categories: biomarkers of disease (measurement of endogenous substances or parameters indicative of critical disease processes) biomarkers of exposure (detection and measurement of exposure to critical drugs and chemicals), biomarkers of response (measures of endogenous substances or parameters indicative of pathological or biochemical changes), biomarkers of susceptibility, biomarkers of prediction for the conversion MCI to AD etc. The actual status of these biomarkers is reviewed, together with the nowadays trends of their further development. The arguments pleading for the usefulness of biomarkers in periphery (blood, urine and saliva), immune and inflammatory (e.g. cytokines, antibodies against amyloid-beta protein etc), related to the oxidative-mediated tissue injury, large number of proteins (proteomics), lipids (lipidomics), or small metabolic substrates (metabolomics), as well as in cortico-spinal fluid, rises also the problem of the establishment of patterns or profiles of MCI markers (signature), the use of panels of markers carefully selected and needing confirmation in independent sets of samples. A special field of highly useful biomarkers actually in full swing is performed by epigenetic attempt of dementia and predementia stage. Due to the role of DNA methylation, histone modifications, and other chromatin-remodeling-mediated gene regulation crucial for high-order cognitive functions such as learning and memory, the development of molecular markers related these events is understood as a prodigious step in the development of further, powerful instruments for MCI detection and AD prediction.
Finally, the presentation concludes the main arguments pleading for the important contribution that the use of biomarkers may provide in the detection of AD predementia, extracted from a meta-analysis of specific reports. Among other, despite the inference of RC Petersen, and
JQ Trojanowski (2009) that to the question of AD biomarkers usefulness we could answer “Potentially Yes for Clinical Trials but Not Yet for Clinical Practice”, a lot of reports suggest that the actual biomarkers may have sufficient accuracy to be used in the AD prodromal phase, highlight the challenges and suggest solutions.
ASYMPTOMATIC CAROTID STENOSIS: INTERVENTION OR JUST STICK TO MEDICAL THERAPY: THE CASE FOR MEDICAL THERAPY
J. Streifler
Neurology Unit, Rabin MC, Petach Tikva & Tel Aviv University, Israel
Asymptomatic significant (≥50%) carotid stenosis (ASCS) is a frequent finding in the aging population. The prevalence of moderate stenosis (50-70%) increases from 3.6% for those less than 70 years to 9.3% in those ≥70 years. The (additional) prevalence of severe (70-99%) stenosis is around 2%.
The natural history of ASCS is quite benign. The overall risk of stroke is around 2% per year and within the group higher degrees of stenosis are associated with higher risks.
Carotid endarterectomy (CEA) has been evaluated in several studies; mainly ACAS and ACST. An overall modest benefit of about 1% risk reduction (per year) was found for CEA (with a peri-operative risk of less than 3%) versus medical treatment, over a five year period.
Basically these 2 studies recruited similar patients with ≥60% stenosis based on carotid Duplex. However, the similar favorable results differ: While ACAS (published in 1995) found the risk for ipsilateral stroke in the medical group to be 11% over a five year period, the 11.8% risk observed in ACST (published in 2004) was for any strokes – showing a better "natural history" for patients with ASCS in the more recent study.
This observation adds to other reports suggesting a better outcome for patients with ASCS in recent years, probably because of better medical treatment, mainly due to the significant increase in the use of statins.
The suggested guideline that results from the above mentioned studies is that CEA should be considered in every patient with significant (≥60%?, ≥70%?) stenosis who has a life expectancy of more than five years (& is less than 75 years?).
Taking this advice as such would mean that we should screen for ASCS and operate on all appropriate candidates. This will result in a surge of CEA's!
Such a recommendation is not in place because the observed benefit of CEA by numbers needed to treat (NNT) per year to prevent any stroke is around one hundred! (for symptomatic patients NNT is less than 10). This high figure (i.e. low yield) results from failure of these studies to identify specific risk factors (including the degree of stenosis within the wide range [60 to 99%] allowed in the studies) in patients with ASCS. Some studies are underway.
Therefore, at present, it seems that for most patients, best medical treatment is the best option. Alternately they should join studies that will help identify patients with the highest risk – those who will clearly benefit from carotid intervention.
NEWLY EMERGING THERAPIES: DO THEIR POTENTIAL BENEFITS OUTWEIGH THE RISKS?
O. Stuve
Department of Neurology, University of Texas Southwestern Medical Center at Dallas, Graduate Program, Department of Immunology, University of Texas Southwestern Medical Center at Dallas, Neurology Section, VA North Texas Health Care System, Medical Service, Dallas, TX, USA
Based on the results of two phase II clinical trials, natalizumab (Tysabri®) was the first monoclonal antibody approved for the treatment of relapsing forms of multiple sclerosis (MS). After its initial approval, three patients undergoing natalizumab therapy in combination with other immunoregulatory and immunosuppressive agents were diagnosed with progressive multifocal leukoencephalopathy (PML). Two of these individuals had a fatal outcome. After a voluntary withdrawal of natalizumab for 14 months and cessation of all clinical trials during this period, the agent was later re-approved, and its use restricted to monotherapy in patients with relapsing forms of MS. Over the past year, seven additional cases of PML were reported in MS patients receiving natalizumab monotherapy. Thus, there is currently no convincing evidence that natalizumab-associated PML is restricted to combination therapy with other disease modifying or immunosuppressive agents.
Our groups recently showed that natalizumab therapy results in a reduction of CD4+ T cells within the cerebrospinal fluid (CSF) that is ten-fold more pronounced than the reduction in the number of CD8+ T lymphocytes. Interestingly, it appears that the effect of natalizumab on cell numbers in the CSF persists for at least 6 months after cessation of treatment. Thus, the biological effects of natalizumab far exceed its pharmacological half-life. We also studied the expression of major histocompatibility complex (MHC) I and II, and the number and phenotypes of leukocytes in cerebral perivascular spaces (CPVS). We observed that natalizumab therapy was associated with a significant decrease in the cell surface expression of MHC class II molecules, and the numbers of dendritic cells in CPVS. In addition, no CD4+ T cells were detectable in this compartment. In a more recent longitudinal and serial cross-sectional assessment, a subgroup of patients was followed over 14 months after cessation of natalizumab. With regard to clinical disease activity, neuroimaging, and immune responses, the majority of patients in our cohort were stable. Decreased lymphocyte cell numbers and altered cell ratios returned to normal 14 months after cessation of natalizumab.
Based on available data, we believe that natalizumab treatment may result in prolonged immunosuppression in peripheral organs, and the delayed onset of adverse events. We also think that our observations may not apply exclusively to natalizumab. A wide group of therapeutic agents that are currently in development of clinic trial decrease the number of immune-competent leukocytes in peripheral organs either by preventing their egress from secondary lymphoid organs, depleting leukcocyte subsets, or preventing their migration across biological membranes. Further adverse infectious and neoplastic events have to be expected unless a balance between immunosuppression and immunoreconstitution can be achieved.
SHOULD PROPHYLACTIC ANTIEPILEPTIC THERAPY BE PRESCRIBED TO ALL PATIENTS WITH BRAIN TUMORS? YES!
T. Tomson
Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Treatment with antiepileptic drugs (AEDs) should be initiated whenever the benefits of treatment outweigh the risks and drawbacks. In the general population, treatment is considered indicated after two or more unprovoked seizures, because the risk of recurrence is high, in the order of 60 % to 80% if untreated. The effects of seizures may be devastating, disrupting normal activities in an unpredictable way, sometimes causing physical injuries and occasionally even leading to death. People with high risks of seizures should as far as possible be protected against such risks. Protection against the additional threat of seizures is particularly important in patients who already have to carry the burden of a serious and often life threatening disease such as a brain tumour. It is a fact that the development of convulsions adds substantial morbidity to patients with brain tumours (1).
Neoplasms account for approximately 6 % of all cases with unprovoked epileptic seizures and are thus a major cause of epilepsy. Patients with brain tumours have a high risk of developing seizures and epilepsy. Seizures are the first presenting symptom or sign of the tumour in 30-50% of the cases (2), and further patients develop epilepsy during the course of the tumour disorder.
The risk of having seizures varies according to pathology and localisation. Low-grade gliomas are among the most epileptogenic tumours; seizures occurring in 60-85 % of patients with low-grade astrocytomas and oligodendrogiolmas. Between 80% and 90% of patients with ganglioglioma develop epilepsy whereas almost all patients diagnosed with dysembryoblastic neuroepithelial tumours will do so. Seizures are slightly less common in association with high-grade gliomas, meningiomas, and in general with brain metastasis, but never the less occur in 25% to 50% of such cases.
A few studies have attempted to assess the effectiveness of prophylactic treatment with AEDs in patients with brain tumours. The results have not been convincing but this may be related to patient selection, study design, as well as the type of AED being assessed. To date, randomized studies have included prophylactic use of phentyoin and phenobarbital in patients undergoing surgery for primary or secondary brain tumours and with no evidence of effectiveness (3). On the other hand, our knowledge of the effectiveness of AEDs in modifying the natural course of epilepsy in general, including non-tumour related cases, is far from complete.
Indications for AED treatment are generally based on an estimation of the risk of further seizures without treatment. It is commonly considered reasonable to initiate treatment after two (or more) unprovoked seizures and this is based on an estimated recurrence risk in the order of 60% to 80% (4). A similarly great, or even greater, risk of seizures is seen in association with many types of brain tumours. For these reasons it is logical to consider prophylactic prescribing of AEDs to all patients with brain tumours.
References: 1.Taphoorn MJ. Neurocognitive sequelae in the treatment of low-grade gliomasSemin Oncol 2003; 30:45-8; 2. van Bremen MSM, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007; 6: 421-30; 3. Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 2001; 42: 515-24; 4. Hauser WA, Rich SS, Lee JR, et al. (1998). Risk of recurrent seizures after two unprovoked seizures. N Engl J Med 338:429-434.
SHOULD VALPROATE BE PRESCRIBED TO WOMEN OF CHILDBEARING AGE WITH IDIOPATHIC GENERALIZED EPILEPSY? YES!
T. Tomson
Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden
Epilepsy is a condition that can have serious consequences. The unpredictable occurrence of seizures with loss of consciousness can lead to limitations in everyday life, career choices, and sometimes result in physical injuries and even in death. Life expectancy is reduced, partly because of the underlying cause of epilepsy, but uncontrolled seizures can also result in Sudden Unexpected Death (SUDEP). Full control of seizures is the best strategy to reduce or eliminate these hazards of epilepsy. This is equally important for women of childbearing age as it is for any other person with epilepsy. In fact, a survey of maternal mortalities over 15 years in the UK indicated that 3.4% of all mortalities during pregnancy were among women with epilepsy. Although the absolute risk was low, 1 in 1,000 pregnancies, it was ten times that expected and as found in the general population (1). Review of the medical records indicated that many deaths were in seizures in women who had withdrawn their treatment out of fear for teratogenic effects of their medication.
SANAD is by far the largest randomized head-to-head comparison of different antiepileptic drugs (AEDs) in newly diagnosed epilepsy. This unblinded trial compared longer term outcomes with valproate, lamotrigine, and topiramate in 716 patients with newly diagnosed generalised or unclassifiable epilepsy (2). For patients with idiopathic generalised epilepsy, valproate was significantly better than both lamotrigine and topiramate in terms of time to treatment failure. Hence, there is no doubt that valproate by far is the best documented, and probably most efficacious treatment for idiopathic generalised epilepsy.
Furthermore, pregnancy registries have indicated that the likelihood of seizure control during pregnancy is higher with valproate than with other drugs such as lamotrigine (3). Control of generalised tonic-clonic seizures is probably particularly important during pregnancy as major convulsive seizures might be harmful also to the foetus.
However, data from pregnancy registries and other observational studies have reported higher malformation rates in offspring of mothers treated with valproate during pregnancy compared with some other AEDs (3-7). Preliminary data also suggest poorer cognitive outcome in children exposed to valproate in utero (1, 8). For this reason, and despite its superior efficacy, valproate is by many considered not to be the drug of first choice for women with epilepsy that are of childbearing potential.
However, this does not mean that valproate should never be prescribed to women with epilepsy of childbearing age. Several studies have demonstrated that the risk of congenital malformations in association with valproate exposure is dose-dependent (3, 6, 8-10). Malformation rates at maternal doses below 800-1,000 mg/day might not be higher than with other AEDs. Similar dose dependency has been observed with adverse effects on post-natal cognitive development. A retrospective study analysing verbal IQ at school age in relation to prenatal exposure to AEDs found no difference compared to other monotherapies with valproate doses below 800 mg/day (1). The prospective NEADs study reported lower IQ in children who had been exposed to valproate in utero compared with phenytoin, carbamazpeine, or lamotrigine (8). However, children exposed to valproate doses <1,000 mg/day did not differ in IQ from those exposed to other AEDs.
In summary, there is no evidence that maternal use of a low dose of valproate, less than 800-1,000 mg/day, is associated with worse pregnancy outcome than any other AED. Such low dosages may be sufficient for many patients, for in particular those with idiopathic generalized epilepsies.
Women of childbearing age are entitled to, and in need of, effective treatment of their epilepsy. In idiopathic generalized epilepsies the alternatives to valproate are few, lamotrigine, topiramate and levetiracetam. Among these, only lamotrigine has a reasonable documentation of relative safety during pregnancy. But this drug is difficult to use in women of childbearing age, because of pharmacokinetic alterations in pregnancy and interactions with contraceptives. In many cases, it is reasonable to first try other alternatives, but if these fail, women of childbearing potential should not be denied the chance of seizure control by use of valproate but always with provision of appropriate counselling.
References: 1. Adab N, Kini U, Vinten J, Ayres J, Baker G, Clayton-Smith J, Coyle H, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004; 75:1575-83. 2. Marson AG, Al-Kharusi AM, Alwaidh M, et al. on behalf of the SANAD Study Group. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for treatment of generalized and unclassifiable epilepsy: An unblinded randomized controlled trial. The Lancet 2007; 369-1016-26. 3. Vajda FJ, Hitchcock A, Graham J, et al. The Australian Register of Antiepileptic Drugs in Pregnancy: the first 1002 pregnancies. Aus NZ J Obstet Gynecol 2007; 47:468–74. 4. Wyszynski DF, Nambisan M, Surve T, et al. Antiepileptic Drug Pregnancy Registry. Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology 2005; 64(6):961-5. 5. Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006; 77(2):193-198. 6. Artama M, Auvinen A, Raudaskoski T, et al. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology 2005; 64(11):1874-8. 7. Wide K, Winbladh B, Kallen B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population -based register study. Acta Paediatr 2004; 93(2):174-6. 8. Meador KJ, Baker GA, Browning N, et al. NEAD Study Group. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009 Apr 16; 360(16):1597-605 9. Samren EB, van Duijn CM, Christiaens GC, et al. Antiepileptic drug regimens and major congenital abnormalities in the offspring. Ann Neurol 1999; 46(5):739-46. 10. Samren EB, van Duijn CM, Koch S, et al.Maternal use of antiepileptic drugs and the risk of major congenital malformations: a joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia 1997; 38(9):981-90.
SOCIAL REINTEGRATION FOLLOWING TRAUMATIC INJURY: THE FRENCH EXPERIENCE
J. L. Truelle, C. Francois-Guinaud, P. North, M. Onillon,
E. Tazopoulou, M. Montrueil
Department of Neurorehabilitation University hospital, Garches, France
Objective: TBI is a specific handicap, often hidden, mainly due to cognitive and, moreover, behavioural sequelae. After initial care and rehabilitation, social re-entry is a long-term, fluctuant and precarious process which is not enough dealt with.
Material and method: Select the most innovative, validated methods and re-entry programmes in France
Results: 1. Ccontinuity of care: bridge the gap between initial rehabilitation and community reintegration via 30 transitional units for evaluation, retraining, social-vocational orientation and follow-up. Their program is based on a multidisciplinary team mainly implemented by neuropsychological, psychological and ecological rehabilitation and lasts for a maximum of 6 months. Since 2005, a new law on handicap promoted a central resource office for disabled people in each of the 100 districts;
2. Assess mental status by TBI-specific evaluation tools, through an international network as a basis for therapeutic project: EBIS holistic
ANIMAL MODELS FAIL US IN FINDING NEW TREATMENTS IN STROKE
J. van Gijn
FRCP (Edin), Utrecht, The Netherlands
Preclinical studies of neuroprotective agents in experimental stroke raised high expectations of clinical efficacy. After all 114 drugs showing such promise turned out to fail in clinical trials, the question arose whether experiments are poor indicators of clinical outcome or whether the potentially best drugs might not have been tested in clinical trials. Therefore, O’Collins et al. (Ann Neurol 2006:59:467-77carried out a systematic search of in vivo and in vitro experiments, with either functional or histological end points. There was no evidence that drugs used clinically (114 drugs) were more effective experimentally than those tested only in animal models (912 drugs). Improvement in focal models, for example, averaged 31.3 +/- 16.7% for the former versus 24.4 +/- 32.9% for the latter, respectively. There was a tendency towards an inverse relationship between the quality of the study and the degree of neuroprotection: as the quality of evidence increased with more Stroke Therapy Academic Industry Roundtable (STAIR) criteria being met, the average size of the neuroprotective effect of drugs tended to regress toward the overall mean of approximately 25%. No relationship was found between mechanism of action and efficacy.
Since at least part of the failure in the transition from experimental to clinical studies in stroke can been attributed to the imprecision introduced by problems in the design of experimental stroke studies, Crossley et al (Stroke 2008;39:929-34) took this further. By means of a so-called meta-epidemiologic approach, they addressed the effect of randomization, blinding, and use of comorbid animals on the estimate of how effectively therapeutic interventions reduce infarct size. They identified 13 meta-analyses that described interventions and outcomes in experimental stroke, involving 15,635 animals. For each meta-analysis, a reanalysis was conducted to estimate the impact of various quality items on the estimate of efficacy, and these estimates were combined in a meta -meta-analysis to obtain a summary measure of the impact of the various design characteristics. Studies that included unblinded induction of ischemia reported effect sizes 13.1% (95% CI, 26.4% to 0.2%) greater than studies that included blinding, and studies that included healthy animals instead of animals with other diseases overstated the effect size by 11.5% (95% CI, 21.2% to 1.8%). No significant effect was found for randomization, blinded outcome assessment, or high aggregate methodological quality score. This empirical evidence of bias in the design of studies, with studies that included unblinded induction of ischemia or healthy animals overestimating the effectiveness of the intervention might account for the failure in the transition from bench to bedside of stroke therapies. In addition, published reports of animal studies often fail to mention important methodological aspects. A review by the CAMARADES group (Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Stroke) found that only 36% of studies reported random allocation to treatment group, only 11% report allocation concealment, and only 29% reported the masked assessment of outcome (Dirnagl, J Cereb Blood Flow Metab 2006;26:1465-78).
Therefore MacLeod et al. (Stroke 2009; 40: e50-2), have proposed a set of requirements for the reporting of experimental stroke studies. They propose that at least the following aspects of study design should be accounted for: 1) description of animals (species, strain, substrain and source); 2) sample size calculation; 3) criteria for inclusion and exclusion of animals; 4) method of randomisation; 5) concealment of allocation; 6) reporting of animals that were initially included but subsequently excluded from the analysis; 7) masked assessment of outcome; 8) conflicts of interest and funding.
To epitomise: animal studies are indispensible as a prelude to clinical trials in patients, but their methods need to be equally rigorous. Mice as well as men deserve better than most studies done so far.
Document, B.N.I. screening of higher cerebral functions, GOS extended and QOLIBRI (TBI QoL measure);
3. Promote specific re-entry programs:
- day centers, to share the burden with family, mobile teams for at home help, sheltered work centers, centers grouping various programs and facilities devoted to TBI in the same area;
- limited medication, ecological neuro-psychological rehab, retraining skills in real life problems, communication peer groups, violence and maltreatment prevention(Ministry of Health); TBI-specific regional networks, academic approach of medico-social field, return to driving, environmental structuration, « re-socialization » in holistic program, case management;
4. Take into account the head-injured family:
- Via a systemic approach, a training;
- The role of 50 local family associations grouped in a national union: UNAFTC linked to the national association of professionals: France Traumatisme Crânien;
- A resource centre in Paris for specific information and training (5 university courses), documents Shaking Baby, Parentality;
5. Early identify the mild TBI at risk: short examination of jockeys in the race course and license withdrawal for a week in case of concussion, information leaflets on MTBI (Ministry of Health);
6. Improve the medico-legal expertise: guidelines for a TBI- specific expert-appraisal, mandatory neuropsychological assessment and family interview, annual forum gathering lawyers and health professionals;
Conclusion: develop information and training, the family role, improve the care of psychological and behavioral troubles, cross the bridge between rehab and re-entry, promote TBI specific holistic programs and networks, and improve compensation via guidelines to evaluate the hidden handicap
STATINS SHOULD BE GIVEN TO EVERY STROKE PATIENT FOR SECONDARY STROKE PREVENTION: NO
J. van Gijn
FRCP (Edin), Utrecht, The Netherlands
HMG- CoA reductase (or 3-hydroxy-3-methyl-glutaryl-CoA reductase or HMGR) is the rate controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMG-CoA reductase inhibitors or statins reduce the level of cholesterol and have proved effective in preventing major vascular events in patients at high risk for such events, especially after stroke or myocardial infarction. In addition, other beneficial actions have been claimed for this class of drugs (‘pleiotropy’), such as restoration of endothelial function, anti-inflammatory effects and regulation of heart rhythm. Consequently, statins are nowadays prescribed for a variety of indications other than prevention of infarcts of the brain or heart: chronic renal failure, reconstructive operations of the aorta or its major branches and chronic lung disease – not to mention far-fetched indications like cancer prevention or erectile dysfunction. I shall limit myself to the evidence for the use of statins in the prevention of stroke.
A systematic review by Amarenco and Labreuche (Lancet Neurology 2009) showed that in patients with previous cerebrovascular disease the relative reduction in the risk of subsequent stroke is around 12% (95% confidence interval 1-22%). This difference is only just statistically significant, but the estimate may have been too conservative, for several reasons. Firstly, the interval between the cerebrovascular event and inclusion in the statin trials was usually several months, even in the only one of the four relevant trials that was specifically aimed at patients with cerebrovascular disease (SPARCL investigators, N Engl J Med 2006), whereas the recurrence risk of cerebral ischaemia is highest in the first few days and weeks. Secondly, there is abundant evidence that also major vascular complications other than stroke are also prevented by statins, in patients with manifest vascular disease (Amarenco and Labreuche, Lancet Neurology 2009) as well as in patients with vascular risk factors (Brugts et al., BMJ 2009). Given that the cost-effectiveness ratio for patients with vascular risk factors has been estimated at € 15 000 per quality-adjusted life year (QALY) for statins and extra antihypertensive agents' together (Kok et al., European Journal of Cardiovascular Prevention and Rehabilitation 2009), the ratio should be even more favourable for patients with a history of stroke, and for statins alone.
Given all this positive news, why not statins for all patients with stroke? What I wish to emphasise are three caveats: the first applies to patients with a history of cerebral haemorrhage, the second to unusual age groups (the very young and the very old), and the last to the problem that with short treatment periods, by poor compliance or other reasons, statins can do more harm than good.
It should not be forgotten that one fifth of all strokes are intracerebral haemorrhages. Cohort studies have repeatedly shown that subjects with relatively low levels of cholesterol have a relatively high risk of intracerebral haemorrhage. The intervention studies with statins have confirmed that the association is causal: independent meta-analyses show that statin treatment in patients with a history of stroke or transient ischemic attacks causes a significant increase in the rate of haemorrhagic stroke (Vergouwen et al., Stroke 2008; Amarenco and Labreuche, Lancet Neurology 2009). In other words, the beneficial effect of statins is greater for ischemic stroke than for total stroke, since part of the advantage in terms of prevented brain infarcts is offset by an excess of haemorrhagic strokes. Therefore statins should not be prescribed to stroke patients unless infarction or at least the absence of a haemorrhagic lesion has been ascertained by brain imaging in the acute stage of the stroke. Also, one should probably not prescribe stains to patients with a history of cerebral haemorrhage preceding the ischemic episode that raises the issue of preventive treatment.
Children and adolescents under 18 years of age have not been included in trials of the secondary prevention of stroke. Therefore the results of these trials cannot be extrapolated to them, despite encouraging results of statins in children with familial hypercholesterolemia. To some extent the same problem arises with the aged. Although the SPARCL investigators found no difference in efficacy between patients below or above 65 years (Chaturvedi et al., Neurology 2009), this does not allow the conclusion that there is no upper age limit. Patients of 80 years or older formed less than 5% of the study population in stroke prevention trials. A study with pravastatin in patients aged 70-82 with vascular risk factors found a reduction of cardiac events, but not of stroke.
Finally, statins commonly produce side effects, especially on muscle. Muscle tenderness develops in over 10% of patients treated with statins (Sathasivam & Lecky, BMJ 2008; Joy & Hegele, Ann Intern Med 2009). Fortunately rhabdomyolysis is rare, occurring in about one in every 7500 patients. Also, against every patient with a serious adverse event there are 7 patients in whom a serious vascular complication is prevented (Silva et al., Clinical Therapeutics 2006). Nevertheless, the benefits of statins do usually not emerge until a few years of treatment, whereas adverse events occur within months (Sathasivam & Lecky, BMJ 2008; Joy & Hegele, Ann Intern Med 2009). In real life about half of patients who are prescribed statins discontinue the medication within a year (Goldenberg & Glueck, Vasc. Heath Risk Manag. 2009), which means that in short term users the disadvantages may well outweigh the advantages. Patients who are known to be non-compliant with medicines are not likely to benefit from statins.
MIGRAINE IS A NEURONAL DISORDER: COMMUNICATION BETWEEN TRIGEMINAL SYSTEM AND TRIGEMINAL NUCLEUS CAUDALIS
L. Vecsei
Department of Neurology, Alber Szent-Gyorgyi Clinical Center, University of Szeged, Szeged, Hungary
Migraine is a disabling disorder that affects more than 10% of the adult population. Concerning the pathomechanism of migraine cortical spreading depression (CSD) is a slowly propagating wave of neuronal depolarization which travels across the cortex and is followed by a long- lasting suppression of neuronal activity. CSD activates the trigeminovascular system in experimental animals, as the migraine aura precedes trigeminally mediated migraine headache in humans. Furthermore, interictal hyper-excitability of the human cortex in migraineurs, suggesting enhanced susceptibility due to a genetic or environmental cause. Administration of nitroglycerol in a migraine model results in an increased number of c-fos-expressing secondary sensory neurons in the caudal trigeminal nucleus. Since synapses between first- and second-order trigeminal neurons are mediated by excitatory amino acids, NMDA receptors are inhibited by kynurenic acid, though this crosses the blood-brain barrier only poorly. In our studies systemic treatment of rats with SZR-72, a newly synthesized kynurenic acid analog, diminishes the nitroglycerol-induced increase of c-fos immunoreactivity in the brain stem, while treatment with kynurenic acid resulted in a smaller decrease. Thus, it appears that SZR-72 treatment moderates the propagation of noxious stimuli in the brain stem, though the effective inhibition of NMDA receptors. Furthermore, kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. Animal and human studies suggest that a critical role of CGRP in migraine is its central action on the second-order neurons in the trigeminal nucleus caudalis and in the medullary dorsal horn that processes nociceptive input from the meninges. In contrast, peripheral actions of CGRP in the meninges, including meningeal vasodilation, are unlikely to play a significant role in the genesis of migraine. Dysfunction in the cortex and/or brainstem is a likely candidate in the pathomechanism of migraine. Brainstem dysfunction may contribute to migraine by directly activating the trigeminal system or facilitating its activation. CGRP acting at CGRP receptors, and glutamate, acting at NMDA receptors, contribute to the transmission of nociceptive impulses from the periphery to the brain.
CLADRIBINE TABLETS FOR THE TREATMENT OF RELAPSING-REMITTING MULTIPLE SCLEROSIS
P. Vermersch
University of Lille, Nord de France, Lille, France
Cladribine is a synthetic deoxynucleoside analog that is resistant to degradation by adenosine deaminase (the enzyme that catalyses the first step in the metabolism of naturally occurring deoxynucleosides). Cladribine acts as a pro-drug; it is activated through phosphorylation by the enzyme deoxycytidine kinase and is inactivated through dephosphorylation by 5’-nucleotidases. Lymphocytes have high levels of adenosine deaminase, high levels of deoxycytidine kinase and low levels of 5’-nucleotidases, compared with other hematological and non- haematological cell types. The resistance of cladribine to adenosine deaminase degradation coupled with the high ratio of deoxycytidine kinase to 5’-nucleotidase in lymphocytes results in a selective accumulation of activated (i.e. phosphorylated) cladribine in lymphocytes, which interferes with DNA synthesis and repair, ultimately causing apoptosis. Treatment with cladribine tablets has been investigated in relapsing remitting multiple sclerosis (RRMS) using a short-course, oral dosing regimen. The treatment aims to offer patients year-long efficacy with once-daily dosing, for only a few days during the year.
The CLARITY study was a phase III, randomized, double-blind, placebo-controlled, multicentre, 96-week study. 1,326 patients with RRMS were randomized 1:1:1 to receive placebo or short courses of cladribine tablets (once daily for 4 or 5 consecutive days) at the start of 2 (3.5 mg/kg cumulative dose) or 4 (5.25 mg/kg cumulative dose) consecutive 28-day periods during the first 48 weeks of the study, followed by 2 additional short courses at Weeks 48 and 52. Primary and secondary efficacy endpoints were met with high levels of statistical significance. Thus, patients treated with cladribine tablets achieved 55– 58% reductions in the annualized relapse rate over the 96-week study period (0.14 and 0.15 vs. 0.33 in the 3.5 and 5.25 mg/kg arms vs. placebo; both p<0.001). Cladribine tablets treatment resulted in more relapse-free patients compared to placebo at Week 96 (79–80% vs. 61%; p<0.001 for both treatment arms vs. placebo) and a 31–33% lower risk of 3-month sustained disability progression over the course of the study (p≤0.026 for both treatment groups vs. placebo). Patients treated with cladribine tablets had significant reductions, compared to placebo, in T1 Gd-enhancing lesions, active T2 lesions and combined unique lesions (p<0.001 for both treatment arms vs. placebo) at all times assessed for MRI activity. The improvements in clinical efficacy and MRI parameters were rapid and sustained over the course of the study. Furthermore, cladribine tablets therapy demonstrated a favorable safety profile. Overall, the frequency of reported treatment-emergent AEs was comparable across treatment groups, with some exceptions related to the mechanism of action of cladribine. In total 7.9% and 3.5% of patients discontinued due to AEs in the 5.25 mg/kg and 3.5 mg/kg arms, respectively, compared to 2.1% in the placebo arm. Lymphopenia occurred at a higher frequency with cladribine tablets, as expected from its mechanism of action. Three malignancies were reported during the study in the cladribine treatment groups, and one in post-study surveillance. These cases were isolated events across different organ systems.
A robust clinical study program is continuing to investigate the potential of cladribine tablets as an MS therapy. The CLARITY EXTENSION study will further assess safety and efficacy of cladribine tablets during a 96 week follow-up for patients completing CLARITY. The ORACLE-MS study (Oral Cladribine for Early MS) is a randomized, double-blind, 96-week phase III efficacy and safety study in patients with an initial clinical demyelinating event who are at high risk of converting to MS. The ONWARD study (Oral cladribine added-ON to interferon beta in patients With Active Relapsing Disease) is a randomized, double-blind, phase II study that will analyze the safety and efficacy of treatment with cladribine tablets + interferon beta over 96 weeks in subjects with inadequate response to interferon beta therapy.
CONFIRMING THE DIAGNOSIS OF EARLY AD/MCI WITH BIOMARKERS IS CLINICALLY USEFUL?
P.-J. Visser
The Netherlands
The concentration of beta amyloid 1-42 and total tau in cerebrospinal fluid (CSF) are excellent markers of Alzheimer’s disease (AD). The place of these markers in the diagnostic evaluation of non-demented subjects with cognitive impairments is still not clear. We will argue that these markers should not be measured as part of the routine assessment of these subjects for the following reasons. First, assessment of these markers will not have therapeutic implications because there is no effective AD therapy available in this group. Second, it is difficult to give patients a prognosis on an individual basis, as previous studies have shown that patients with abnormal CSF markers can remain stable for many years.
IS SPECT IMAGING CRITICAL TO DIAGNOSE DEMENTIA WITH LEWY BODIES?
Z. Walker
FRCPsych, University College London, UK
Dementia with Lewy bodies (DLB) is a common form of dementia. The characteristic features of DLB are progressive dementia particularly affecting attention, visuo-spatial and executive ability, fluctuating cognition, spontaneous parkinsonian symptoms, persistent vivid visual hallucinations, hypersensitivity to neuroleptic medication and REM sleep behavioral disorder.
Patients with DLB frequently have mixed pathology. The presence of Alzheimer’s disease (AD) pathology modifies the clinical features of DLB, making it harder to distinguish DLB from AD clinically during life, with AD being the main differential diagnosis. Clinical diagnostic criteria for DLB applied at presentation can fail to identify up to 50% of cases. Over the last 10 years there has been a major change in the attitude of clinicians and the public - it is not sufficient any more to make a diagnosis of “dementia”. The requirement is now to make an accurate diagnosis of DLB as this influences treatment, management and prognosis.
A correct diagnosis of DLB helps careers to understand the different symptom profile of patients with DLB, particularly vivid visual hallucinations, episodes of increased confusion and reduced alertness. Patients with DLB are at high risk of developing neuroleptic sensitivity (50% of cases, given a neuroleptic, will develop severe parkinsonism, increased confusion, worsening hallucinations and even coma). Correct diagnosis of DLB alerts the clinician that apart from cognitive impairment patients frequently exhibit motor symptoms, psychiatric features, sleep disorder and autonomic dysfunction. DLB patients have a less good response to L-dopa compared to patients with Parkinson’s disease. They are more prone to developing psychosis when treated with anti- parkinsonian medication, but have a good response to cholinesterase inhibitors. They have greater impairment of everyday activities than AD cases and need more resources. Finally, failure to diagnose DLB may affect AD trials.
At present there are several imaging techniques that can improve the identification of DLB during life. In this presentation the role of dopamine transporter (DAT) will be discussed. Patients with DLB, compared to AD, have a pronounced pre-synaptic dopaminergic deficit in the striatum. At present the most studied technique in DLB for assessing dopaminergic pathways is 123I-FP-CIT SPECT, although other techniques such as DTBZ PET have also yielded promising results. Following a number of single centre studies1-3 of 123I-FP-CIT SPECT which used both semi-quantitative and visual analysis there is now good evidence from an autopsy study and a European multicenter study that 123I-FP-CIT SPECT has high sensitivity and specificity for distinguishing probable DLB from non-DLB dementia4,5. The autopsy study is on-going and additional results continue to support the published data and will be presented. Follow-up data from the European Trial also indicate that 123I-FP-CIT SPECT is helpful in clinically less clear cases of possible DLB6.
References: 1. Walker Z, Costa DC, Walker RWH, Shaw K, Gacinovic S, Stevens T et al. Differentiation of dementia with Lewy bodies from Alzheimer's disease using a dopaminergic presynaptic ligand. J Neurol Neurosurg Psychiatry 2002; 73:134-140. 2. Ceravolo R, Volterrani D, Gambaccini G, Rossi C, Logi C, Manca G et al. Dopaminergic degeneration and perfusional impairment in Lewy body dementia and Alzheimer's disease. Neurol Sci 2003; 24(3):162-163. 3. O'Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol 2004; 61(6):919-925. 4. McKeith I, O'Brien J, Walker Z, Tatsch K, Booij J, Darcourt J et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007; 6(4):305-313. 5. Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry 2007; 78(11):1176-1181. 6. O'Brien JT, McKeith IG, Walker Z, Tatsch K, Booij J, Darcourt J et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry 2009; 194(1):34-39.
SUBSTANTIA NIGRA HYPERECHOGENICITY IS A RISK MARKER FOR PD (NO)
U. Walter
Department of Neurology, University of Rostock, Germany
In patients with Parkinson’s disease (PD) an increased echosignal (‘hyperechogenicity’) of the substantia nigra (SN) is typically found on transcranial sonography (TCS). A number of independent studies have demonstrated that this TCS abnormality can be detected in about 90% of PD patients. The degree of SN hyperechogenicity in an individual PD patient was found to be stable during the disease course in a five-year follow-up study. This, together with the finding of marked SN hyperechogenicity also in about 9% of healthy adult subjects, has led to the idea that TCS might be helpful for detecting individuals who are at risk, or already at pre-motor stages, of PD. This idea is supported by the results of several cross-sectional clinical and radiotracer imaging studies that were reviewed recently. These studies have shown that SN hyperechogenicity in adults without a movement disorder is associated with a subclinical malfunction of the nigrostriatal dopaminergic system. Moreover, SN hyperechogenicity was related to subtle motor asymmetry in non-depressive and, even more frequently, in depressive subjects.
However, the question of whether the TCS finding of SN hyperechogenicity alone is a risk marker for PD should be currently answered with NO for the following reasons:
• First, the results of ongoing large longitudinal studies dealing with this issue are not yet available.
• Second, it can be inferred from the prevalence differences of SN hyperechogenicity in healthy adults (9%) and of PD in subjects older than 60 years (1-2%) that at most 10-20% of healthy subjects with SN hyperechogenicity will develop PD during their life. Even if taking into account that more subjects may reach pre-motor stages of PD, the value of TCS alone in predicting the occurrence of PD symptoms will be limited.
• Third, TCS findings do not represent a correlate of the progressive nigrostriatal neurodegeneration. SN hyperechogenicity is rather thought to reflect increased amounts of iron in the SN. Even though brain iron metabolism is likely to be involved in the pathogenesis of PD, its precise contribution to the risk of PD has not yet been established. Consequently, in this context the predictive value of SN TCS findings remains unclear.
• Fourth, SN hyperechogenicity is a frequent finding not only in PD but also in some other neurodegenerative diseases with Parkinsonism such as dementia with Lewy bodies and corticobasal degeneration. In these entities, it is unclear yet at which time point in the disease course the TCS abnormality emerges. If SN hyperechogenicity in these disorders is present already at presymptomatic stages this might contribute to a lower diagnostic specificity.
Based on the current evidence it appears reasonable to consider the TCS finding of SN hyperechogenicity as a marker for nigrostriatal vulnerability. The value of SN TCS alone for predicting specifically an increased risk of PD, however, remains unclear. It needs to be elucidated in further studies which co-factors cause the development of PD in individuals with SN hyperechogenicity.
Nevertheless, for the detection of individuals at risk of developing PD, TCS might be useful in a stepwise approach, applying TCS primarily as a screening tool in epidemiologically defined populations at risk. Further diagnostic steps of such an approach may include clinical, genetic, biochemical and other neuroimaging investigations. Consequently, several longitudinal TCS studies have been initiated since 2004 to assess the value of SN hyperechogenicity in combination with other TCS, clinical and neuroimaging findings in predicting an individual high risk of PD.
References: Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K. Degeneration of substantia nigra in chronic Parkinson's disease visualized by transcranial color-coded real-time sonography. Neurology 1995; 45: 182–184; Berg D, Godau J, Walter U. Transcranial sonography in movement disorders. Lancet Neurol 2008; 7: 1044–1055; Berg D, Merz B, Reiners K, Naumann M, Becker G. Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson's disease. Mov Disord 2005; 20: 383–385; Berg D, Becker G, Zeiler B et al. Vulnerability of the nigrostriatal system as detected by transcranial ultrasound. Neurology 1999; 53: 1026–1031; Ruprecht-Dörfler P, Klotz P, Becker G, Berg D. Substantia nigra hyperechogenicity correlates with subtle motor dysfunction in tap dancers. Parkinsonism Relat Disord 2007; 13: 362–364; Walter U, Hoeppner J, Prudente-Morrissey L, Horowski S, Herpertz SC, Benecke R. Parkinson's disease-like midbrain sonography abnormalities are frequent in depressive disorders. Brain 2007; 130: 1799-1807; Spiegel J, Hellwig D, Möllers MO et al. Transcranial sonography and [123I]FP-CIT SPECT disclose complementary aspects of Parkinson's disease. Brain 2006; 129: 1188- 1193; Snyder AM, Connor JR. Iron, the substantia nigra and related neurological disorders. Biochim Biophys Acta 2009; 1790: 606-614; Rhodes SL, Ritz B. Genetics of iron regulation and the possible role of iron in Parkinson's disease. Neurobiol Dis 2008; 32: 183-195; Walter U, Dressler D, Wolters A, Wittstock M, Greim B, Benecke R. Sonographic discrimination of dementia with Lewy bodies and Parkinson's disease with dementia. J Neurol 2006; 253: 448-454; Walter U, Dressler D, Wolters A, Probst T, Grossmann A, Benecke R. Sonographic discrimination of corticobasal degeneration vs progressive supranuclear palsy. Neurology 2004; 63: 504–509; Berg D. Ultrasound in the (premotor) diagnosis of Parkinson's disease. Parkinsonism Relat Disord 2007; 13 (Suppl 3): S429-433; Walter U. Transcranial brain sonography findings in Parkinson's disease: implications for pathogenesis, early diagnosis and therapy. Expert Rev Neurother 2009; 9: 835- 846.
CIDP CAN BE TREATED PERMANENTLY WITH IVIG (PRESENTING THE CON SIDE)
I. Wirguin
Israel
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is a progressive or relapsing remitting acquired disorder, affecting the peripheral nervous system and gradually leading to increasing disability. A significant body of circumstantial evidence suggests an immune mediated pathogenesis in CIDP, but to date, putative auto-antigens are still unrecognized and an appropriate animal model has not been developed. Thus, in the absence of sensitive and specific biomarkers, the diagnosis of CIDP depends on clinical and electrodiagnostic criteria, supported only by a laboratory finding of increased cerebrospinal fluid protein levels. The diagnostic criteria for CIDP have, therefore, been the subjected to repeated consensus conferences resulting in the formulation of a set of complex electrodiagnostic requirements for diagnosing CIDP. Currently, it seems that these criteria, which are helpful in the conduct of clinical trials, might be too limiting for the actual clinical setting and may miss many patients who might otherwise benefit from the currently available therapies.
Therapy in CIDP is also mostly not strictly evidence based. A number of randomized controlled studies, mostly on a rather small scale, have shown short term beneficial effects of corticosteroids, plasma exchange or intravenous immunoglobulins (IVIg). The response rate to any of these treatment modalities appears to be around 60-70%, and there is a practically absolute lack of evidence to support any of the suggested "2nd line" treatments for non-responders.
The main drawback of these clinical trials is their short term of treatment attempt, compared to the chronic nature of the disease. In one single recent trial (the "ICE" study), a special formulation of IVIg was evaluated for 24 weeks with a blinded trial extension of another 24 week period. Efficacy of IVIg-C was maintained through this study period, in which rather high IVIg maintenance doses were administered (1 g/kg every 3 weeks). While this seems promising, a year is still a short term compared to the clinical course of CIDP, and the cumulative cost of these high doses over many years would become prohibitive.
Beyond the strictly "evidence based" arena it would seem reasonable to select IVIg for long term therapeutic trials because of its more favorable side effect profile compared to steroids and relative ease of administration compared to plasma exchange. This has prompted many neurologists involved in CIDP patient care to attempt prolonged IVIg treatment, using various dosage and interval regimens following a favorable initial response to the standard loading dose of 2 g/kg. Indeed, many of these attempts seem successful and many patients appear to be doing well for some years on long term IVIg maintenance therapy.
However, consolidating these personal experiences into a statement that: "CIDP can be treated permanently with IVIg" is an overly ambitious leap, and the following arguments against such a statement should be made and carefully considered:
1. True long term treatment of CIDP with IVIg has never been rigorously tested in a randomized trial. Furthermore, the dosing and interval regimens have not been worked out and neither has the possible long term toxicity, so that evidence based recommendations for CIDP cannot be formulated at this time.
2. The major drawback of IVIg is its prohibitive costs when administered over an extended number of years. Admittedly, some patients benefit from relatively low maintenance doses, bringing the actual cost to the range of current multiple sclerosis therapies, but doses like those given in the ICE study are associated with costs beyond the ability of most health care systems. Therefore, rigorous cost-benefit analysis data should be obtained in order to make a viable recommendation regarding IVIg treatment in CIDP.
3. The initial response rate to IVIg is around 60%. Once subjected to long term therapy, this rate undergoes further attrition due to relapses or side effects, so that in the long range less than half of the patients are expected to benefit from IVIg therapy, leaving the majority of the patients without an efficacious solution.
4. The pathogenesis of CIDP and the mechanisms of actions of IVIg are still inadequately understood. Further intense research is needed to elucidate these questions and pave the road to the development of more efficacious therapies which may benefit a larger cohort of the patient. Statements such as: "CIDP can be treated permanently with IVIg" might deflect research funds to other areas and slow the necessary progress towards better therapies.
HSV-1 AS THE CAUSATIVE AGENT OF BELL'S PALSY (PRESENTING THE PRO SIDE)
I. Wirguin
Israel
Peripheral facial nerve palsy occurs with an approximate annual incidence of 35-40 per 100,000 people. About one third of these palsies are caused by recognizable causes such as trauma, basilar meningitis, sarcoidosis, and diabetes and ear infections. Of special note is the Ramsay-Hunt syndrome, in which reactivation of the Varicella Zoster Virus (VZV) causes severe unilateral facial nerve palsy and sometimes deafness, associated with the characteristic vesicular eruption on the tympanic membrane or the hard palate. In the remaining two thirds of the patients, the etiology is not recognizable, and thus they are diagnosed as Bell’s palsy.
A prevailing theory implicates Herpes Simplex Virus-1 in the etiology of Bell’s palsy. Several lines of evidence lend support to this theory:
1. The Ramsay-Hunt syndrome which serves as proof of concept that viral reactivation in or near the facial nerve structures can cause peripheral facial nerve palsy.
2. HSV-1 genomic DNA is detected in a high percentage (up to 86%) of geniculate ganglia tested at autopsies, with a much higher viral load than that seen in the trigeminal ganglia.
3. HSV-1 DNA was isolated from epineural fluid from 11/14 Bell’s palsy patients undergoing decompressive surgery of the facial nerve. Similar findings were demonstrated regarding VZV DNA in Ramsay-Hunt syndrome patients. Furthermore, HSV-1 DNA can be detected by PCR from saliva of Bell’s palsy patients during the acute phase but not from controls.
4. A mouse model has been developed in which auricular inoculation of HSV-1 causes facial paralysis in 60% of the animals, and HSV-1 can be demonstrated by immunofluorescence in the geniculate ganglion, and demyelination of the descending motor fibers of the facial nerve develops in parallel to the paralysis.
Thus there appears to be ample evidence for the presence of HSV -1 in the geniculate ganglia and for reactivation during the acute phase of facial paralysis. The rodent model and the VZV precedence of viral etiology for paralysis further support the HSV-1 hypothesis.
The major argument against the herpes theory is based on the lack of therapeutic efficacy of anti-viral agents with established anti-HSV-1 activity in two large scales, recently conducted, randomized clinical trials. However, a putative model, in which HSV-1 reactivates within the sensory somata in the geniculate ganglion, evoking a local inflammatory response which causes dysfunction and demyelination of the adjacent motor fibers within the confines of the narrow bony canal, would explain this discrepancy. The HSV-1 reactivation is probably self limited and at its peak by the time that paralysis develops, and thus the anti-viral agents, unlike corticosteroids would fail to affect the clinical course of the affliction.
Two other points that still need to be reconciled with the HSV-1 hypothesis and which probably reflect the biological differences between various sensory ganglia include the lack of mucosal eruption in the area supplied by the sensory component of the facial nerve, (unlike that seen in trigeminal HSV-1 reactivation of herpes labialis), and the low recurrence rate of Bell’s palsy compared to herpes labialis.
BIOMARKERS OF NEURODEGENERATION: APPLICATION AND IMPLICATION
D. Woitalla
Department of Neurology, Ruhr-University of Bochum, St. Josef Hospital, Bochum, Germany
Biomarkers are biologic parameters reflecting the presence or the severity of a disease. According to the definition, they might reflect the central pathogenic processes of the disease, but this is not mandatory.
The expectations on biomarkers are high, as they supposed to
• improve diagnostic quality,
• disclose the underlying pathogenetic process,
• help to monitor the disease,
• develop novel drug therapies,
• determine the efficacy of novel drug therapies
It is of tremendous interest to develop biomarkers for neurodegenerative diseases as these diseases develop over several years. In this context the clinical manifestation present the endpoint of a long lasting pathological process.
The search for biomarkers may be hampered by the fact, that most of the clinically defined diseases reflect heterogeneous pathogenic processes resulting in relative uniform clinical pictures. In contrast, monogenetic diseases coding a specific pathogenetic substrate, which can be detected with “genetic biomarkers”, identify in contrast to any expectation, only a statistical risk, to develop a specific phenotype.
So far genetic studies of monogenetic diseases fail to demonstrate the ability of a genetic biomarker to predict the onset or course of a specific disease. The interaction between genetic factors and the environment seems to play a key role in the manifestation of a disease, thus the biomarker “genetic mutation” reflects more a risk than a disease.
Most of the clinical needs which are addressed to a biomarker are related to the assessment of disease severity and progress. Taken the results of genetic studies into account, it seems reasonable to look for “non-geneti biomarkers”, which might interact with the genome, but could also interact with the proteins (the genetic product) or the sequel of its functional loss. Cell loss determines the end of the cascade of neurodegeneration and it seems reasonable to follow factors indicating the cell loss. Therapeutic strategies will focus much more on the process of neurodegeneration, irrespective of the pathogenetic cause of a disease. Even more the efficacy of therapeutic strategies will rely on its capacity to influence an individualized degenerative process. This was shown in Alzheimer's disease, where immunization targeting Abeta does not appear to have major effects on tangle pathology. In this regard it seems reasonable to look for “proteomic biomarkers” reflecting the cell degeneration and as a consequence the conversation of a risk to a disease. As a matter of course this means the ability to detect a phenotype before its clinical presentation. For example this was demonstrated for Abeta deposits, which could be demonstrated in Alzheimer's dementia 10- 15 years prior to clinical onset.
Biomarkers have to fulfil criteria of specificity and sensitivity along with the ability to detect an unspecific degenerative process. Keeping in mind the limited humoral armentarium of cell destruction, the combination of a specific disease related biomarker and an unspecific, progression related, biomarker could overcome the limitations of hitherto known biomarkers for neurodegenerative diseases.
CLINICAL ASPECTS OF PSEUDOBULBAR AFFECT AND CLINICAL TRIALS, INCLUDING STAR
D. Wynn
Consultants in Neurology, Northbrook, IL, USA
Introduction: Pseudobulbar affect (PBA) is a condition characterized by uncontrollable and inappropriate outbursts of laughter and/or crying that are incongruent with the patient’s underlying emotional state. PBA is always associated with an underlying neurologic disorder or injury and occurs frequently in patients with multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), stroke, traumatic brain injury, and Alzheimer’s disease. The prevalence of PBA in MS is approximately 10% and is more common in patients with greater cognitive and physical impairment.
The pathophysiology of PBA is not well defined but is believed to be related to lesions that interrupt neural circuits connecting the cortex, cerebellum, and brainstem. The neurochemical basis of PBA may include dysfunction of glutamatergic, dopaminergic, serotonergic, and other transmitter systems.
The toll taken by PBA on psychological, social, and physical function should not be overlooked. PBA is a source of extreme embarrassment that may lead to social isolation. It may also impair rehabilitation efforts and feeding or care needs related to the underlying condition. A description of a crying episode by a 55 year old patient with PBA emphasizes the impact of PBA on daily life:
“You have no idea how terrible it is when the crying is fully triggered and takes hold like a seizure. I can not control any of it. I simply disintegrate and it is not only emotionally horrible with me, it is physically painful and debilitating.
Despite the negative impact of PBA on patients’ lives, it is often unrecognized or misdiagnosed.
Diagnosis of PBA: Diagnostic criteria for PBA have been proposed in an effort to facilitate recognition of PBA, reduce variability in diagnosis, and improve evaluation of treatment.
Essential Criteria
1) Patient has episodes of involuntary or exaggerated emotional expression (laughter, crying, or related emotional displays) resulting from a brain disorder
• Episodes represent a change from the person’s usual emotional reactivity
• Episodes may be incongruent with mood or exaggerated
• Episodes are independent or in excess of any provoking stimulus
2) The disturbance causes clinically significant distress or impairment in social or occupational function.
3) Symptoms are not better accounted for by another neurologic or psychiatric disorder.
4) Symptoms are not the direct physiologic effect of a substance (ie, drug of abuse or medication).
Supportive Features
1) Autonomic changes (eg, facial flushing)
2) Pseudobulbar palsy signs (eg, increased jaw jerk, tongue weakness, dysarthria)
3) Proneness to anger
Descriptive Characteristics
1) Sudden onset of episodes
2) Brief duration of episodes (seconds to minutes)
3) Variable severity among patients, similar frequency and severity within individual patients
4) Episodes are stereotypical both in their appearance across various neurologic disorders and within a patient
The most critical step in the diagnosis of PBA is differentiating it from depression. Key distinguishing characteristics indicative of PBA (versus depression) include: incongruence of mood with emotional display, brief duration of episodes, involuntary control of episodes, stereotypical presentation, provoked by minimal or no stimulus, and episodes may be accompanied by anger or frustration, but not thoughts of worthlessness, hopelessness, or guilt. Additionally, fatigue, appetite changes, sleep disorders, and anhedonia are not associated with PBA.
Clinical Trials of PBA in MS Patients: Few well-controlled trials have evaluated the treatment of PBA and only one placebo-controlled trial in MS has been published. Panitch and colleagues evaluated a combination of dextromethorphan (DM) and quinidine (Q) in a multicenter, double-blind, parallel group study. DM is an uncompetitive NMDA receptor antagonist and a sigma-1 receptor agonist and reduces excitatory neurotransmission through interaction with these receptors. Q is a potent inhibitor of CYP450 2D6 activity and is used to block the extensive metabolism of DM that would otherwise occur.
In this trial, patients with MS were randomized (1:1) to receive DM/Q (30 mg of each; n = 76) or placebo (n = 74) bid for 12 weeks. Baseline scores on measures of PBA severity were similar between groups. For the primary outcome, change from baseline in Center for Neurologic Study Lability Scale (CNS-LS) score, the adjusted mean reduction in the DM/Q group was significantly greater than in the placebo group (7.7 versus 3.3, P < 0.0001). The mean number of crying and laughing episodes per week was also significantly lower in the DM/Q group compared with placebo (4.7 versus 11.5, P = 0.0002).
DM/Q was well-tolerated. The overall adverse event incidence rate was 81.6% in the DM/Q group and 85.1% in the placebo group. Adverse -event-related withdrawals occurred in 14.5% of the DM/Q group and 10.8% of the placebo group. The most common adverse events reported by a greater number of DM/Q-treated patients were dizziness, nausea, and fatigue. Headache was the most common event overall but was reported at nearly twice the incidence in placebo-treated patients (29.7% versus 15.8% for DM/Q).
The promising results from the Panitch et al. trial led to the evaluation of additional dose formulations of DM/Q for PBA (the STAR trial). This 12-week, multinational, double-blind, placebo-controlled, parallel-group study evaluated 326 patients with PBA (129 with MS and 197 with ALS) treated with DM/Q 30/10 mg, DM/Q 20/10 mg, or placebo. The primary outcome was change from baseline in the daily rate of laughing and crying episodes from the patient diary. Secondary efficacy outcomes included changes from baseline in CNS-LS scores and Pain Rating Scale scores (MS patients only). Efficacy and safety results from the total population and the MS subset will be reported during the presentation.
References: Arciniegas DB, Lauterbach EC, Anderson KE, et al. The differential diagnosis of pseudobulbar affect (PBA). Distinguishing PBA among disorders of mood and affect. Proceedings of a roundtable meeting. CNS Spectr. 2005; 10:1-14; quiz 15-16. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005; 17:447-454. Cummings JL, Arciniegas DB, Brooks BR, et al. Defining and diagnosing involuntary emotional expression disorder. CNS Spectr. 2006; 11:1-7. Panitch HS, Thisted RA, Smith RA, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006; 59:780-787. Pope LE, Khalil MH, Berg JE, Stiles M, Yakatan GJ, Sellers EM. Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J Clin Pharmacol. 2004;44:1132-1142.
BE CAREFUL OF CAA AND MICROBLEEDINGS WHEN GIVING ANTI-THROMBOSIS TREATMENT
W.W. Zhang
Department of Neurology, PLA of Beijing Military General Hospital, Beijing, China
As every one recognize that thrombolytic therapy by using rt-PA for acute ischemic stroke may cause intracerebral hemorrhage. But we don’t know if anti- platelet aggregation medicine such as Aspirin, Clopidograle, Cilostazol, Wafaring, could cause micro bleeds or not since we give so many anti- platelet agents to vascular risk factors. There is increasing evidence that CAA could be a risk factor for micro bleeds and hemorrhagic lacunars. If we give anti-platelet agents for stroke prevention to them, it can be more danger than without medicine prevention.
Leucoariaosis and aging over 80-years are also including in stroke prevention group. They may more already have micro bleeds before we put anti-platelet agents. They, of cause, easy to get micro bleed in the cerebral.
In our Cilostazol clinical trail, we reported 720 ischemic strokes, 24% with microbleeds before giving aspiring or cilostazol. 12 months later, 6 symptomatic cerebral hemorrhages which including in 5/6 hemorrhagic lacunars. And 5 asymptomatic new cerebral hemorrhages suggest those agents could cause micro bleeds. Another study was undergoing is about 36 over 80-year-old cerebral infarctions with MRI/T2* to investigate micro bleedings. 21/36 was found to be microblles; 27/36 was larcunarns.
In conclusion: Be careful of those CAA and hemorrhagic lacunars.
MECHANICAL EMBOLECTOMY
M.J. Abelson
The Heart Unit, Vergelegen Medical Clinic, Somerset West, South Africa
Mechanical embolectomy, using the Merci Retriever, has been shown to be effective in the management of acute large vessel occlusion strokes (Merci and MultiMerci trials) up to 8 hours after symptom onset. In South Africa, and world wide, there are very few interventional neuro- radiologists. The acute stroke interventions using the Meri retriever are performed by the cardiologist at The Vergelegen MediClinic, Somerset West, and South Africa. To date mechanical embolectomy has been performed on 16 patients with successful reperfusion on 15 (94%). The outcome of these patients far exceeds the expected natural history of large vessel occlusive strokes. See tables 1 & 2 attached. A cardiologist, who is an experienced carotid interventionalist, can safely and effectively perform acute stroke
interventions with little additional training. Patients presenting with
devastating large vessel occlusion strokes can therefore be offered
mechanical embolectomy should standard thrombolysis fail or be
contra-indicated (the vast majority of these patients).
Acute stroke patients taken to the cath lab at Vergelegen Medi
Clinic Jan 2007 to May 2009 N=21
• Male 9 (42%)
• Age 68.6y (41-96y)
• AF 10 (48%)
• Duration 4.9h(2-10)
• NIHSS 21.6 ( 8 – 40)
• Merci used 16 (76%)
• Recanalization 15 (94%)
• Adjunctive IC tPA 10-20mg 7 (33%)
• Culprit Vessel
- ICA-T 10 (48%)
- MCA 8 (38%)
- Basilar 1 (5%)
- Normal 2 (10%)
• Outcome when merci used N = 16
-MRS*0 1 (6%) *MRS 0-6 where 0=normal
-MRS 1 2 (12%) and 6 = dead
-MRS 2 6 (38%) 0 – 2 good outcome
-MRS 3 1 (6%)
-MRS 4 0
-MRS 5 1 (6%)
-Mortality 5 ( 31%) (post CPR,96y,81y,79y,67y old)
• Outcome when no merci used N = 5
-Mortality 1(20%) (86y old)
-MRS < 2 0
-MRS 3 2 (40%)
-MRS 4 1 (20%)
-MRS 5 1 (20%)
CURATIVE TREATMENT OF ALZHEIMER DISEASE: CLOSE PERSPECTIVE OR DREAM?
P. Giannakopoulos
Department of Psychiatry, University Hospitals of Geneva, Switzerland
The perspective of curative treatments for Alzheimer disease (AD) based on the inhibition or clearance of AB protein marked a turn in late 90s. Despite the initial enthusiasm, recent studies pointed to the limited efficacy of these strategies when they are addressed to patients with clinically overt dementia. This was the case for PBT2, a metal-protein attenuating compound affecting the toxic oligomerization of AB but also for active immunization. The increasing concern about the presence of irreversible damage that may prevent a positive response to disease- modifying strategies even in mild AD cases gave raise to the concept of mild cognitive impairment (MCI).
Unfortunately, MCI is a late diagnosis at least from a biological viewpoint. AD pathology is estimated to begin at least a decade before the appearance of any cognitive symptom with impairments becoming clinically detectable and a 60% loss of pyramidal neurons in layer II of the entorhinal cortex is already present in MCI cases. Not surprisingly, new concepts have been developed in a tentative to define earlier stages of the dementing process such as the pre-MCI.
Although of interest, this concept is not yet validated and is limited by the a posteriori judgment that it implies.
Moving away from an overoptimistic conception of AD therapy, new evidences suggest that future curative treatments may be mainly successful in elderly individuals without cognitive symptoms at very high risk to develop subtle cognitive changes. The combination of
genetic, neuroimaging and biochemical markers are mandatory to address this complex issue.
REHABILITATION MODEL IN EXPERIMENTAL MEDICINE: IS AN EARLY FUNCTIONAL IMPROVEMENT AFTER TRAUMATIC BRAIN INJURY POSSIBLE?
M. Lippert-Gruener, O. Svestkova
University of Cologne, Cologne, Germany and Charles University Prague,
Czech Republic
Introduction: The present study was designed to determine whether exposure to multisensorical early rehabilitation model (MRM) after moderate traumatic brain injury (TBI) in rats would promote the recovery of neuromotor function superior to that under standard
conditions (SC).
Materials and Methods: A total of 28 Sprague-Dawley rats were randomized to one of the following groups: 1) injured/MRM (n = 12); 2) sham/MRM (n = 2); 3) injured/SC (n = 12); 4) sham/SC (n = 2).
Under anesthesia, animals were subjected to either a moderate fluidpercussion injury (2, 1 atm) or to a sham-injury. Thereafter the injured/MRM and the sham/MRM groups were placed together into specially modified custom made cages (three large cages connected via tunnels) containing various types of bedding and stimulating objects, e.g. balls, robes, running wheel etc.. Along with environmental complexity the animals underwent a specific protocol of motor and multisensoric rehabilitation model. In contrast, the injured/SC and the sham/SC groups were returned to their standard cages where they were housed individually without stimulation. Motor function was assessed by using a composite neuroscore (NS) test
battery at 24h, 7, and 15 DPI.
Results and conclusions: Neuromotorfunction assessed by NS was markedly reduced in both injured groups at 24h post-injury being nonsignificant. However, animals in the injured/RM group performed significantly better when tested for neuromotorfunction as compared to injured/SC animals on 7d and 15d DPI (7d: p = 0,005; 15d: p < 0,05). Conclusion: These results provide experimental evidence that postoperative exposure of rats to multisensoric rehabilitation model (MRM) is associated with significant improvements in the recovery of
neuromotorfunction function after TBI. Whether these improvements correlate with reduced CNS scar formation is currently under investigation.
ANOSOGNOSIA IN DEMENTIA: A BLESSING AND A CURSE
L. Liss
Department of Neurology, Ohio State University, Columbus Ohio USA
The behavior of each patient who has Alzheimer type dementia can be placed on a spectrum that spans the two polar extremes. On one side are those who are happy, placid, friendly and cooperative, which contrast with those who are intense, angry and scared and they are diagnosed as “behavioral problems”
Patients with dementia display unpredictable levels of awareness of their cognitive deficits which ranges from complete awareness to anosognosia.
The challenging symptoms are in patients who are oblivious to their lost abilities, which may include among others, driving, and operation of electronics, tools and various skills which were previously mastered. Those patients are the unwilling and resisting subjects to our attempts to assure their safety.
The positive effect of anosognosia can be observed in individuals who are either unaware or unaffected by their deficits. They remain unperturbed even when challenged and represent the group which is very easy to provide care for since they remain pleasant and cooperative.
The other extreme are the patients with acute awareness in the early stages of the disease and a dim, but disturbing “awareness” in the latter stages. They are the ones who lash out, pace, yell, are verbally and physically abusive or are in terror which causes severe agitation.
The clinical significance: Discontinuation of therapy of currently used Alzheimer medications can significantly reduce behavioral problems in patients with moderate and advanced dementia.
Supported in part by Ohio State University Development Fund “Alzheimer Initiative” # 306246
IS BOTULINUM TOXIN INJECTION A COST- EFFECTIVE THERAPY FOR CHRONIC MIGRAINE? PRELIMINARY RESULTS
J. Rothrock, C. Scanlon, D.A. Andress-Rothrock
University of Alabama College of Medicine, Birmingham, Alabama, USA
Background: Chronic migraine (CM) is both prevalent and responsible for a disproportionate share of the direct and indirect costs associated with migraine generally. Until recently there existed no treatment of clearly demonstrable value for suppressing CM, but preliminary results from the PREEMPT study indicate that botulinum- type A (BOT-A) injections may be safe and effective when administered for that purpose.
Objective: To determine whether treatment with BOT-A may be associated with a reduction in direct costs attributable to CM Methods: We used open-label BOT-A according to the PREEMPT study protocol to treat a series of CM patients presenting to a university-based headache clinic. Along with the patients’ clinical responses, we assessed the costs of emergency department (ED) utilization over the 6 months preceding and following initiation of treatment with BOT-A.
Results: Of the 83 patients treated, 34(41%) experienced a 50% or greater reduction in headache days per month during the 5th treatment month relative to the pre-treatment baseline month. The gross charges associated with BOT-A therapy totaled $334,060(US).
Over the 6 months following initiation of treatment, the reduction in gross charges attributable to ED utilization totaled $386,456(US. (Conclusion These data suggest that the consequent reduction in direct medical costs related only to decreased ED utilization is sufficient to offset the costs of BOT-A therapy for all patients so treated (i.e., responders and non-responders.
GLUCOCEREBROSIDASE AND PARKINSON DISEASE: GENETIC AND THERAPEUTIC IMPLICATIONS
E. Sidransky, M.A. Nalls, O. Goker-Alpan
Medical Genetics Branch, National Human Genome Research Institute, NIH,
Bethesda, MD & Laboratory of Neurogenetics, National Institute of Aging, NIH,
Bethesda , MD USA
Several lines of research demonstrate a relationship between mutations in glucocerebrosidase (GBA), the gene implicated in Gaucher disease, and the development of parkinsonism. First, patients with Gaucher disease who are homozygous for mutations in GBA have an increased propensity to develop Parkinson disease and related synucleinopathies. Furthermore, carriers' relatives of patients with Gaucher disease have an increased rate of parkinsonism.
Subsequently, investigators from centers around the world screened cohorts of patients with parkinsonism for GBA mutations and found that overall, subjects with Parkinson disease as well as other Lewy body disorders have a significantly higher frequency of GBA mutations. An international multicenter collaborative study of over 5000 patients with Parkinson disease and an equal number of controls demonstrated that in subjects with Parkinson disease the odds ratio for carrying a GBA mutations is greater than 5, rendering the most significant Parkinson risk factor identified to date. In addition, neuropathologic studies of subjects with parkinsonism carrying GBA mutations demonstrate Lewy bodies, depletion of neurons of the
substantia nigra and involvement of hippocampal layers CA2-4. While the basis for this association has yet to be elucidated, evidence continues support the role of GBA as a Parkinson risk factor across different centers, synucleinopathies and ethnicities. Further studies of the association between Gaucher disease and parkinsonism will stimulate new insights into the pathophysisology of the two disorders, and will prove crucial for both genetic counseling of patients and family members and the design of relevant therapeutic strategies for specific patients with parkinsonism.
BACTERIAL VIRUSES BREAK DOWN LEWY BODIES IN AN ANIMAL MODEL OF PARKINSON’S DISEASE: A NOVEL THERAPEUTIC AVENUE
B. Solomon
Tel Aviv University, Tel Aviv, Israel
Parkinson’s disease (PD) is linked to aggregation of misfolded asynuclein, suggesting that disrupting such aggregates may arrest and/or reverse progression of PD. We propose a novel approach involving intranasal administration of filamentous bacteriophages for disaggregation of PD-related protein. Bacteriophages, viruses that infect bacteria, are the most numerous life forms on earth, and their natural contact with human beings is not incidental but rather constant and intensive. Filamentous phages (Ff) are well understood both at structural and genetic levels. Their nanotubular appearance, 900 nm long and 7 nm narrow, consists of a singe-stranded (ss) DNA genome packaged with coating proteins. Phages have a high affinity for and ability to disrupt these sheet structures, including those composed of a-synuclein. We previously demonstrated that the linear structure of filamentous phages confers permeability to the CNS. Here we describe the modulating effect of Ff on aggregation of a-synuclein in a cellular and/or animal model of Parkinson’s disease (PD). ELISA measurement of intracellular a- synuclein soluble oligomers in SHSY5Y cells showed reduced levels of a-synuclein oligomers in phage treated cells compared to non-treated cells. Intranasal administration of Ff to Tg mice model of PD led to considerable reduction of Lewy bodies in the hippocampus and cortex following 8 weekly treatments.
No adverse effects were shown in peripheral organs and blood. The therapeutic potential of phage in PD stems from their unprecedented ability to access the CNS without causing adverse effects in the brain and periphery, and for their lack of natural tropism for mammalian cells.
QEEG-NEUROMETRIC ANALYSIS GUIDED NEUROFEEDBACK TREATMENT IN DEMENTIA 9 CASES: HOW NEUROMETRIC ANALYSIS IS IMPORTANT FOR THE TREATMENT OF DEMENTIA AS WELL AS DIAGNOSIS?
T. Surmeli, A.M.S Ertem
Living Mental Health Center for Research and Education, & Brain Power
Human Resources, Consultancy and Neurotherapy, Istanbul, Turkey
According to DSM-IV, The development of multiple cognitive deficits manifested by both 1-memory impairment (impaired ability to learn new information or to recall previously learned information) and 2- one or more of the cognitive disturbances such as aphasia, apraxia, agnosia and disturbance in executive functioning. In this study we wanted to show the outcomes of a clinical case series using QEEG and neuro-feedback in the assessment and treatment of dementia.
Evaluation measures included QEEG analysis with NX Link data bank, Mini Mental State Exam and clinical interview with the patient and the family. In Neurometric Qeeg analysis, all Qeeg variables are calculated as Z-scores which means -/+2 standard deviation for age is normal. Our hypothesis was that patients who normalize z scores on their Qeeg will most benefit from neuro-feedback treatment. Lexicor Qeeg signals were sampled at 128Hz. Each session was 30 minutes duration, with 1-2 sessions per day. The mean number of sessions completed by the subjects is 120 sessions within 45 days to 90 days Electrode sites for training were selected based on the QEEG(Nx Link) analysis We studied with 9 patients (3 male, 6 female) ranging 45-78. All of the patients showed improvement according to CGI, Mini Mental State Exam, QEEG -Neurometric Analysis and patient’s family interview.
This is a study providing evidence that neuro-feedback treatment can produce improvements in patients with Dementia. Based on our positive results, it is recommended that further control group research be conducted which includes additional outcome measures.
THE “CONTINUUM” OF A UNIFIED THEORY OF DISEASES
G. Vithoulkas
University of the Aegean, Mytilene, Greece
Through the life of a person, from birth to death, there is a "continuum" in the sequence and an underlying connection of the diseases acute and chronic that is affecting each particular organism.
When acute diseases are not properly treated, the overall state of health of the individual is compromised and a chronic condition may start manifesting.
If acute diseases are incorrectly treated and therefore suppressed, will continue in a modified form, as a kind of sub-acute inflammatory process, triggering the expression of the genetic predispositions of the organisms and thus manifesting the chronic degenerative diseases. It appears that all chronic conditions have an inflammatory character and that an "inflammation process" constitutes a common parameter for all diseases.
If the organism, while running a high temperature, is repeatedly stressed in an aggressive way through strong chemical drugs, the immune system, being already in a weakened state, may be finally compromised to such a degree that it may not be able anymore to react by producing a high fever, even if exposed to virulent microbes.
One of the best examples is the chronic fatigue syndrome, called also "post viral syndrome". Another example is given by the acute viral hepatitis that continues as a hepatic derangement and finally cirrhosis, as well as the acute rheumatic fever which ends in chronic heart condition.
The question that here can be raised is: “What is the relation between acute inflammatory processes and chronic diseases characterized by acute exacerbations?”, e.g. M/S or epilepsy. |